2290
A Multiparametric Functional Map for Monitoring and Predicting Chronic Lung Transplant Rejection
Hooman Hamedani1,2, Stephen J Kadlecek1, Ryan Baron1, Faraz Amzajerdian 1,2, Kai Ruppert1, Ian Duncan1, Yi Xin1,2, Mehrdad Pourfathi1, Sarmad Siddiqui1, Luis Loza1, Tahmina Achekzai1, Maurizio Cereda1,3, and Rahim R. Rizi1
1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 3Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, PA, United States
1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 3Anesthesiology and Critical Care, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
mPRM analysis allowed us to distinguish otherwise healthy transplanted lungs and to classify functional abnormalities in lungs compromised by CLAD.
Hypothesis:
Despite lung transplantation’s increasing success rate as a treatment for end-stage lung disease, recipients remain at risk of developing chronic lung allograft dysfunction (CLAD), which remains difficult to diagnose in its early satges1. CLAD was originally diagnosed based on an “FEV1 (forced expiratory volume in 1 second) and/or FVC (forced vital capacity) ≤ 80% baseline function for ≥ 3 weeks”2, although distinct phenotypes of chronic rejection are now recognized, each with unique pathology and histopathological findings.3 Recently, a drop below 90% of a post-transplant FEV1 baseline , coupled with a drop in mid-expiratory flow rates (FEF25-75) below 75% of baseline, have been adopted as the criteria for an initial diagnosis of CLAD3. Since underlying etiology can affect treatment, a robust functional imaging tool would seem well-suited to the dual tasks of diagnosing and monitoring CLAD, particularly one possessed of the ability to differentiate between different etiologies better than global spirometric measures, which cannot. Here, we attempted to take advantage of hyperpolarized (HP) xenon-129 (HXe) MRI’s unique capacity to sensitively assess lung function in order to develop predictive imaging markers capable of identifying CLAD before Pulmonary Function Tests (PFTs) detect a diagnostically significant functional decline. Using a previously developed multi-breath acquisition sequence4, we simultaneously collect sensitive measurements of apparent diffusion coefficient (ADC)—a direct measurement of microstructural alveolar sizes—specific ventilation (SV)5, and oxygen uptake (R)6, all of which can be accurately quantified at an acinar or sub-acinar resolution.Method:
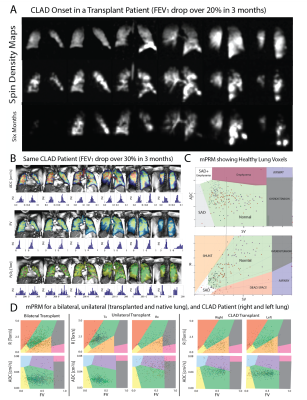
Using a previously developed4 multi-breath protocol consisting of seven normoxic wash-in breaths of HXe gas (shown in Figure 1A), we can regionally measure three interrelated parameters of respiratory function (FV, R, and ADC). Plotting the voxel values on a 3D multi-parametric plane5 (mPRM) containing estimates of FV, R and ADC, we can comprehensively describe respiratory function in different lung regions (Figure 1C). The voxels labeled in each region can then be translated back to their coordinate in order to reconstruct functional maps of the altered lung. HP data collected from 100+ subjects with/without abnormalities were used with a semi-supervised support vector machine classification methodology to classify these distinct regions (Figure 1C). We have previously shown that a combination of these markers can successfully predict future lung function deterioration before PFTs in smokers at risk6. In this longitudinal study, we will monitor the subclinical alterations in transplant recipients by tracking the voxels in different mPRM regions every six months. A model to predict CLAD in the same manner as6 will be tested and compared with existing PFT criteria.Results:
To this point, imaging has been performed in 6 healthy subjects and 5 transplant recipients (1 healthy bilateral, 2 healthy unilateral and 2 diagnosed with CLAD). One of the CLAD subjects has completed a second visit (6 months; Figure A). HXe-derived mPRMs showed that healthy transplant subjects (no FEV1 post-surgical maximum) appeared similarly asymptomatic to controls (most voxels in the green region), while there was significant heterogeneity and elevated ADC in the native lung of unilateral transplant subjects and the right lung of one CLAD subject—consistent with emphysematous lung tissue identified via clinical CT measurements. Figure 1B shows the functional maps in multiple slices across the lung in the representative CLAD subject. Figure 1D shows three representative mPRMs for a bilateral, unilateral and CLAD subjectConclusion:
mPRM analysis allowed us to distinguish otherwise healthy transplanted lungs and to classify functional abnormalities in lungs compromised by CLAD.Acknowledgements
No acknowledgement found.References
1- Tissot A. et al., Front Immunol. 2019; 10: 1681.
2- Verleden G. et al., J Heart Lung Transplant. 2014;33(2):127–33.
3- Gauthier, J. M., et al., Current transplantation 2016. reports, 3(3), 185–191.
4- Hamedani H., et al., J Magn. Reson. In Med., 2016.
5- Hamedani H. et al., American Journal of Respiratory and Critical Care Medicine 2017;195:A5163
6- Baron R., et al., Academic Radiology Volume 26, Issue 3, March 2019, Pages 383-394