2275
Automated mitral valve vortex ring extraction from 4D flow MRI: impact of segmentation1Department of Radiology, Medical University of Graz, Graz, Austria, 2Institute of Medical Engineering, Graz University of Technology, Graz, Austria, 3Computer Vision Center, Universitat Autònoma de Barcelona, Barcelona, Spain, 4Department of Internal Medicine, Medical University of Graz, Graz, Austria, 5Research and Development, Siemens Healthcare Diagnostics GmbH, Graz, Austria
Synopsis
Automated mitral valve vortex ring evolution analysis from magnetic resonance 4D flow data is feasible. However, time-consuming manual segmentation of the left ventricular blood pool represents a bottleneck. We simulated speed-up and variability of manual segmentation and analyzed the impact on vortex ring parameters. Automated mitral valve vortex ring extraction and analysis yielded robust results, even when applying the end-diastolic segmentation mask to all cardiac phases. Error analysis of vortex ring parameters showed that under-segmentation of the ventricular blood pool should be avoided. Speeding up segmentation by using only the end-diastolic mask enables clinical mitral valve vortex ring analysis studies.
Introduction
The mitral valve (MV) vortex ring is a three-dimensional swirling blood flow structure forming during diastole that is assumed to store kinetic energy1 and redirect blood entering the left ventricle (LV) towards the aorta.2,3 It was shown that the temporal evolution of the MV vortex ring can be extracted from time-resolved three-directional cardiovascular magnetic resonance phase-contrast imaging (4D flow).4,5 However, vortex ring evolution analysis is limited by time-consuming manual segmentation of the LV cavity throughout the cardiac cycle, which is either performed directly on 4D flow magnitude images or on balanced steady-state free precession (b-SSFP) images of a separate scan.6 Aim of this study was to evaluate the sensitivity of automated MV vortex ring extraction5 and vortex ring parameters to speeding up LV segmentation by reducing the number of short-axis slices on the one hand and using the end-diastolic segmentation mask for all phases on the other hand, as well as to under- and over-segmentation of the LV blood pool.Methods
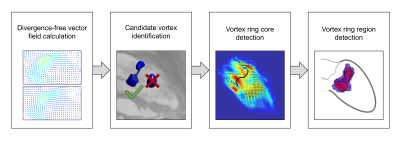
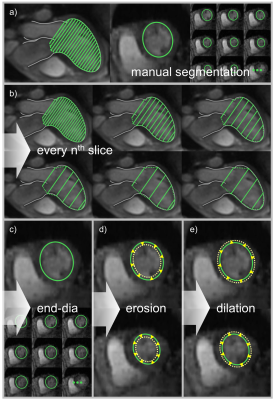
13 subjects (male/female 6/7; age 58.3±6.1 years) underwent 4D flow imaging at 3T (Magnetom Skyra, Siemens Healthcare, Erlangen, Germany). A stack of slices covering the LV was acquired using a two-dimensional retrospectively-ECG-gated phase-contrast sequence with three-directional velocity encoding (temporal resolution 41 ms interpolated to 30 cardiac phases, echo time 3.1 ms, flip angle 12-20°, voxel size 1.8x2.5x4 mm3, velocity encoding 100 cm/s, two-fold averaging). Prototype software (4DFlow, Siemens Healthcare, Erlangen, Germany) was used for pre-processing of velocity vector fields (phase offset error correction, phase unwrapping) and manual LV segmentation on reconstructed short-axis magnitude images (slice distance d = 3.4 mm). Automated extraction and analysis of MV vortex ring evolution were performed by in-house software5 implemented in Matlab (MathWorks Inc., Natick, MA), which included detection of vortex ring core and region (Figure 1) as well as calculation of vortex ring volume, mean vorticity, absolute kinetic energy and relative kinetic energy (normalized by vortex ring volume) for each phase. To assess the sensitivity of MV vortex ring analysis to LV segmentation, three experiments using different segmentation masks were performed (Figure 2). First, the slice distance of short-axis segmentation was increased step-wise by taking each second (2d), third (3d), fourth (4d), fifth (5d) and sixth slice (6d), respectively, leading to a maximum slice distance of 20.4 mm. Second, the segmentation mask at end-diastole (maskend-dia) was applied to all cardiac phases. Third, the segmentation masks of all phases were eroded (maskerode) and dilated (maskdilate) by one voxel, respectively. Pair-wise comparisons of all segmentation masks with the fully segmented control condition regarding vortex ring existence at each phase were performed using kappa statistics. In vortex ring phases common to both segmentation masks, overlap ratios of vortex core and vortex region, respectively, were assessed using the dice coefficient. Early and late diastolic MV vortex ring peak parameters determined under control condition and with each segmentation mask were compared using paired t-test, considering p < 0.05 as significant.Results
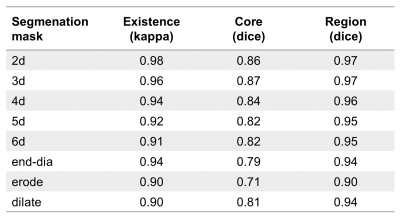
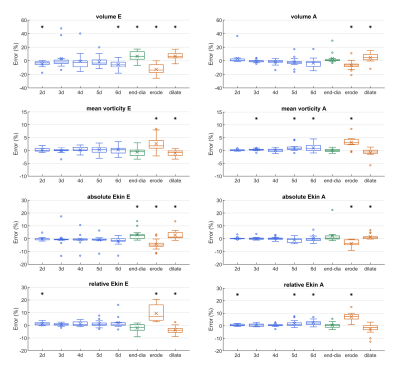
856±140 short-axis slices per subject were manually segmented in the control condition. Early and late diastolic MV vortex rings were detected in each subject for all segmentation approaches. Comparisons with the control condition regarding vortex ring existence and overlap of vortex core and region, respectively, are shown in Table 1. All segmentation masks yielded almost perfect agreement for vortex ring existence and, apart from maskerode, very good to excellent vortex ring core and region overlap ratios. Increasing slice distance yielded a slight decrease in comparison scores. Figure 3 shows error plots for early and late diastolic peak vortex ring volume, mean vorticity, and absolute and relative kinetic energy for all segmentation masks. Most significant differences to the control condition were observed for maskerode and maskdilate, with higher absolute mean errors (up to 13%) for maskerode. While the other segmentation masks also yielded sporadic statistically significant differences, their absolute mean errors were small (<6.8% for peak volume, <1.1% for peak mean vorticity, <3.2% for peak absolute kinetic energy and <2.5% for peak relative kinetic energy).Discussion
Automated MV vortex ring extraction is robust with respect to fast manual segmentation approaches and is less sensitive to over- than to under-segmentation. Increasing the segmentation slice distance not only showed that a speed-up of segmentation on 4D flow magnitude images is feasible, it also indicated that using registered LV contours from b-SSFP short-axis cine images, which are typically acquired with approximately 10 mm slice distance, might work for automated MV vortex ring extraction. If LV segmentation is performed only at one end-diastolic phase, further substantial speed-up can be achieved. maskend-dia yielded only a slightly lower vortex core overlap ratio than the ones of the slice distance experiment while excellently agreeing with the control condition regarding vortex ring existence and vortex region. Furthermore, vortex ring parameters determined with maskend-dia showed low absolute mean errors. The finding that under-segmentation yields larger errors than over-segmentation is reasonable as in the former case parts of vortex ring core and region might be missed in the calculations.Conclusion
Reducing LV segmentation to one end-diastolic phase enables clinical MV vortex ring analysis studies with large patient cohorts. Under-segmentation of the LV blood pool should be avoided.Acknowledgements
We thankfully acknowledge the support of the OeNB Anniversary Fund (Grant No. 17934), the Generalitat de Catalunya (Grant No. 2019FI_B1_000198) and the ESOR fellowship 2018/19.References
1. Hong GR, Pedrizzetti G, Tonti G, et al. Characterization and quantification of vortex flow in the human left ventricle by contrast echocardiography using vector particle image velocimetry. JACC Cardiovasc Imaging. 2008;1(6):705–17.
2. Kilner PJ, Yang GZ, Wilkes AJ, et al. Asymmetric redirection of flow through the heart. Nature. 2000;404(6779):759–61.
3. Pedrizzetti G, La Canna G, Alfieri O, et al. The vortex - an early predictor of cardiovascular outcome? Nat Rev Cardiol. 2014;11(9):545–53.
4. Töger J, Kanski M, Carlsson M, et al. Vortex ring formation in the left ventricle of the heart: analysis by 4D flow MRI and Lagrangian coherent structures. Ann Biomed Eng. 2012;40(12):2652–62.
5. Kräuter C, Reiter U, Schmidt A, et al. Objective extraction of the temporal evolution of the mitral valve vortex ring from 4D flow MRI. Proc Intl Soc Mag Reson Med 27. 2019;1970.
6. Arvidsson PM, Kovács SJ, Töger J, et al. Vortex ring behavior provides the epigenetic blueprint for the human heart. Sci Rep. 2016;6(1):22021.
7. Hunt JCR. Vorticity and vortex dynamics in complex turbulent flows. Trans Can Soc Mech Eng. 1987;11(1):21–35.
Figures