2273
Respiratory-Controlled Adaptive 4D Flow of the Aorta: Reproducibility and Comparison to 2D Phase Contrast Imaging1The University of Melbourne, Melbourne, Australia, 2Austin Health, Heidelberg, Australia, 3The Florey Institute of Neuroscience and Mental Health, Melbourne, Australia, 4Siemens Healthcare Pty Ltd, Melbourne, Australia, 5Siemens Medical Solutions, Chicago, IL, United States
Synopsis
A four-dimensional phase-contrast magnetic resonance imaging sequence with respiratory-controlled adaptive k-space reordering (ReCAR-4DPC) offers potential benefits of improved scan efficiency and motion robustness. Imaging was performed on 15 volunteers and 2 patients with aortic dissection. Inter-scan repeatability and inter-reader agreement of flow metrics derived from ReCAR-4DPC was assessed and compared to metrics derived from 2-dimensional phase contrast imaging (2DPC). There was almost perfect inter-scan and inter-reader concordance (Lin's concordance correlation coefficient for all metrics > 0.91 and >0.98 respectively). Concordance with 2DPC was also high (LCCC all > 0.873). RC-4DFlow is reproducible and repeatable, with high concordance with 2DPC metrics.
Introduction
Aortic dissection is a potentially fatal disease and four-dimensional phase contrast imaging (4DPC) helps quantify aortic haemodynamics for improved monitoring and treatment1. In the thoracic aorta, imaging times can be long when performed with respiratory navigation. Respiratory-controlled adaptive k-space reordering 4DPC (ReCAR-4DPC) can improve scan efficiency and robustness to motion by acquiring the centre of k-space at end-expiration and outer k-space during inspiration2. We evaluate the inter-reader, intra-reader and inter-scan agreement of flow metrics within the thoracic aorta derived from ReCAR-4DPC and compare these to reference standard 2-dimensional phase contrast imaging (2DPC)-derived metrics.Method
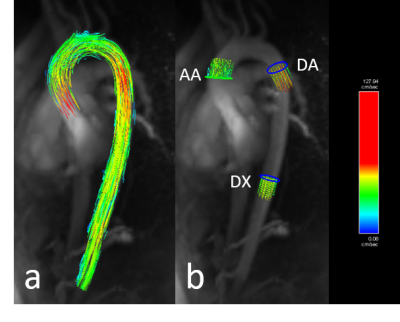
15 healthy volunteers (9F, 6M, range 23-47y, mean heart rate 71bpm) and 2 patients (2M, 74 and 79y, mean 68bpm) with aortic dissection were recruited and imaged on a 3T MRI system (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany). All PC imaging was performed following 0.2 mmol/kg injection of gadobutrol (Gadovist, Bayer).ReCAR-4DPC was performed in the oblique sagittal orientation using a 4D flow prototype gradient echo sequence with retrospective ECG-gating: TR/TE 4.85/2.3ms, temporal resolution 38.8 ms, FA 15°, voxel size 2.4 x 2.4 x 2.5mm3 (interpolated), 40 slices, FOV 380 x 285, GRAPPA 2, navigator acceptance window 8mm. ReCAR-4DPC was acquired twice in 14/17 subjects during the same examination. 2DPC breath hold conventional phase-contrast gradient echo imaging was performed at the ascending aorta (AA), descending aorta (DA) and diaphragm (DX): TR/TE 4.85/2.66ms, temporal resolution 38.8ms, FA 20°, 1.4 x 1.4mm2 pixel size, 5mm slice thickness, GRAPPA 3, TA~14s. 30 cardiac phases were reconstructed for both ReCAR-4DPC and 2DPC. Velocity encoding (VENC) of 150-200cm/s (subject-dependent) was selected for both ReCAR-4DPC and 2DPC, with VENC of 50cm/s in one patient with low aortic velocities. ReCAR-4DPC scan time was recorded.
After training, Reader 1 (R1, no experience with cardiovascular MRI) and Reader 2 (R2, 14 years’ experience) assessed all anonymized ReCAR-4DPC datasets for peak systolic velocity (PSV), average flow (AF) and net forward volume (NFV) using prototype software (4D Flow Demonstrator, Siemens Healthcare, Erlangen, Germany) at AA, DA and DX, matching 2DPC acquisitions. R1 assessed all initial ReCAR-4DPC acquisitions twice to assess intra-reader agreement, and R2 assessed both initial and repeat ReCAR-4DPC acquisitions in 14 subjects for inter-scan repeatability. 2DPC metrics were measured by R2 on a commercial workstation (Multimodality Workplace, Siemens Healthcare, Germany), at least 1 month prior to ReCAR-4DPC reads.
Intra-reader agreement, inter-reader agreement, inter-scan repeatability and concordance with 2DPC derived metrics (all segments combined) was assessed with Lin’s concordance correlation coefficient (LCCC) and reduced major axis regression, enabling analysis for both fixed and proportional biases3.
Results
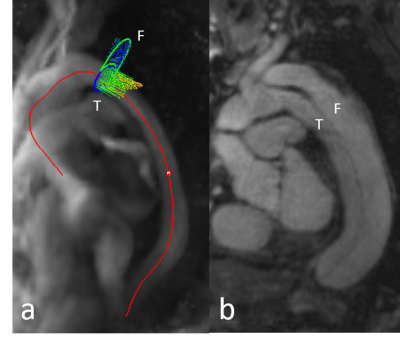
ReCAR-4DPC and 2D PC imaging acquisitions were successful in all subjects, with flow metrics able to be derived from all subjects (representative images in Figure 1 and Figure 2). Average ReCAR-4DPC acquisition time for 17 subjects was 9:57 min (range, 5:55–14:33 min), with mean±SD acceptance rate of 72.65±13.4%. Mean±SD of PSV, AF and NFV derived at each level are provided in Table 1, with true lumen results used for patients. For the two patients, analysis of the false lumen at DA revealed a mean PSV of 47.14 ± 7.93 cm/s for ReCAR-4DPC and 65.41 ± 22.44 cm/s for 2DPC.The reproducibility and 2DPC comparison analysis for flow metrics are summarized in Table 2. There was excellent intra-reader agreement (LCCC all ≥0.987) and excellent inter-reader agreement (LCCC all ≥0.979) for PSV, AF and NFV. There was high inter-scan repeatability for PSV, AF and NFV (LCCC 0.965, 0.907 and 0.981 respectively).
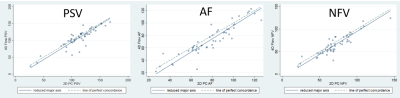
There was near-perfect agreement with 2DPC for PSV, AF and NFV (Table 2 and Figure 3). LCCC was 0.89 for AF, and 0.87 for both PSV and NFV. Reduced major axis regression analysis demonstrated no proportional bias (slope close to 1) and small fixed biases for PSV, AF and NFV, with slightly lower ReCAR-4DPC values compared to 2DPC.
Discussion
Accurate and highly reproducible results were achieved with ReCAR-4DPC, within clinically acceptable acquisition times of approximately 10 minutes. Whilst more rapid techniques are in development2, 4, the employed ReCAR-4DPC prototype is easily implemented and does not require intensive computational post-processing, lending itself readily to clinical use. Small differences to 2DPC derived PSV, AF and NFV were observed, similar to prior experience5. The slightly lower flow metrics observed with ReCAR-4DPC could relate to lower in-plane spatial resolution leading to inaccuracies in luminal segmentation, or slight differences in location/plane of AA, DA and DX samples between the two techniques. Limited results from false lumen analysis were less accurate, likely due to relatively low false lumen velocities.Limitations of the study include small subject number, and relatively poorer in-plane spatial resolution of ReCAR-4DPC, although our parameters conform to consensus guidelines6. The selected VENC may not have accurately captured patient haemodynamics, and a VENC scout could allow for improved VENC selection. A dual-VENC approach may also enable more accurate measurement of false lumen haemodynamics7.
Conclusions
ReCAR-4DPC is an accurate and relatively fast approach for comprehensively measuring the flow metrics throughout the thoracic aorta, exhibiting high reproducibility and robust correlation with conventional 2DPC. Recruitment of a greater number of subjects, including more patients with aortic dissection for further technique validation, and complementary CFD simulations8 are planned.Acknowledgements
This work was supported by funding from the Royal Australian and New Zealand College of Radiologists.References
1. Clough RE, Waltham M, Giese D, et al. A new imaging method for assessment of aortic dissection using four-dimensional phase contrast magnetic resonance imaging. Journal of vascular surgery. 2012 Apr 1;55(4):914-23.
2. Ma LE, Markl M, Chow K, et al. Aortic 4D flow MRI in 2 minutes using compressed sensing, respiratory controlled adaptive k‐space reordering, and inline reconstruction. Magnetic resonance in medicine. 2019 Jun;81(6):3675-90.
3. Ludbrook J. Linear regression analysis for comparing two measurers or methods of measurement: but which regression?. Clinical and Experimental Pharmacology and Physiology. 2010 Jul;37(7):692-9.
4. Bollache E, Barker AJ, Dolan RS, et al. k‐t accelerated aortic 4D flow MRI in under two minutes: feasibility and impact of resolution, k‐space sampling patterns, and respiratory navigator gating on hemodynamic measurements. Magnetic resonance in medicine. 2018 Jan;79(1):195-207.
5. Bollache E, van Ooij P, Powell A, et al. Comparison of 4D flow and 2D velocity-encoded phase contrast MRI sequences for the evaluation of aortic hemodynamics. The international journal of cardiovascular imaging. 2016 Oct 1;32(10):1529-41.
6. Dyverfeldt P, Bissell M, Barker AJ, et al. 4D flow cardiovascular magnetic resonance consensus statement. J Cardiovasc Magn Reson Imaging 2015; 17: 72.
7. Schnell S, Ansari SA, Wu C, et al. Accelerated Dual-venc 4D flow MRI for neurovascular applications. J Magn Reson Imaging 2017; 46: 102-114.
8. Stalder AF, Liu Z, Hennig J, et al. Patient specific hemodynamics: combined 4D flow-sensitive MRI and CFD. InComputational Biomechanics for Medicine 2011 (pp. 27-38). Springer, New York, NY.
Figures