2266
Higher frame-rate phase-contrast with temporally under-sampled phase-reference acquisition: preliminary data in two vascular regions1Department of Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 2Department of Radiology and Biomedical Imaging, Yale, New Haven, CT, United States
Synopsis
Phase-contrast (PC) MRI requires at least doubled scan time compared to anatomical imaging. This has required careful trade-offs regarding spatial and temporal resolution, and breath-holding times, and often the temporal or spatial resolution is insufficient. Here we introduce a simple novel method which temporally under-samples acquisition of the phase-reference images, providing increased temporal resolution. This method is evaluated both in the aortic and pulmonary vein flow, which exhibits several rapid flow peaks requiring good spatial resolution. This method allowed to accurately identify velocity peaks for the aorta and pulmonary veins, providing higher temporal resolution as compared to the standard PC-MRI.
Introduction
Phase-contrast (PC) MRI requires at least doubled scan time (i.e. both velocity-encoded and phase-reference k-space), compared to anatomical imaging. This has required careful trade-offs regarding spatial and temporal resolution, and breath-holding times, and often the temporal or spatial resolution is insufficient. Acceleration methods are critical, and many acceleration methods exist for PC, including, parallel imaging, half-Fourier, under-sampled radial1, and compressed sensing2. Here we introduce a simple novel method for increased temporal resolution, which temporally under-samples acquisition of the phase-reference data, with the hypothesis that the phase reference k-space data is less temporally dynamic, compared to flow-encoded data. We tested the method in two applications: aortic flow, which exhibits rapid systolic flow, and is routinely evaluated by MRI; and pulmonary vein flow, which exhibits two rapid systolic peaks, not easily distinguished, and flow reversal during the atrial kick that is also a rapid phenomenon. Pulmonary vein flow is important, as it is a biomarker of left atrial (LA) pressure and function, and diastolic function3. We evaluated the impact of increased temporal resolution provided by the under-sampled phase-reference method, compared to a standard acquisition.Methods
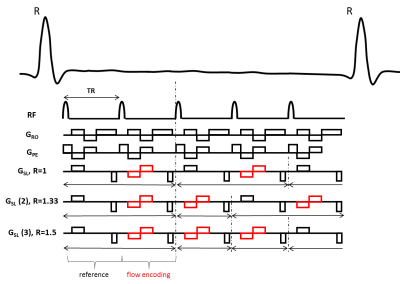
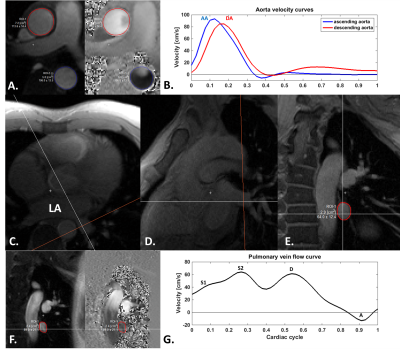
Imaging.Figure 1 presents the method used for accelerated PC, which acquires the phase-reference k-space data less often within the cardiac cycle (R=1.33 or R=1.50 shown), compared to the flow-encoded data. Four patients (26±3.74 years, 2 females) were scanned in a 3T (Siemens Prisma) magnet. Retrospectively ecg-gated PC was acquired for both the aorta and the pulmonary veins during breath hold with the following parameters: FOV/matrix/slice-thickness=340mmx256mm/256x192/6mm, TR/TE/flip-angle/bandwidth=4.3ms/2.43ms/20°/698 Hz/pix. The sequence used parallel imaging with a GRAPPA factor of 2, asymmetric echoes and a pixel resolution of 1.3x1.3mm during 10 heartbeats. A through-plane VENC of 150cm/s was used for the aorta and 80cms/s for the pulmonary veins. Different views per segment (vps) of 4 and 6 were used, resulting in 34ms to 52ms temporal resolution for standard imaging. The effective temporal resolution for the under-sampled acquisition was 25 and 39ms. Both conventional and under-sampled PC were acquired two times to account for test-retest variability. Pulmonary veins were localized using: 1) an axial ecg-gated single-phase GRE sequence of the left atrium, 2) this same sequence was repeated breath held oriented in the pulmonary vein longitudinal direction and 3) in the pulmonary vein orthogonal direction (Figure 2CDE).
Data analysis.
Image reconstruction was processed using MATLAB, and included GRAPPA reconstruction and POCS processing for asymmetric echoes. The reconstruction of the under-sampled PC acquisition used the closest in time pair of flow reference and flow encoded k-space acquisition to compute the phase difference. Flow data analysis was processed using Segment v2.2 R7056 (http://segment.heiberg.se)4. The conventional flow data were extracted for the ascending (AAo) and descending aorta (DAo) (peak systolic velocity, Figure 2B); flow in the pulmonary vein was extracted for peak early systolic S1, peak systolic S2, peak early diastole D and reversed atrial induced velocity peak A (Figure 2G).
Results
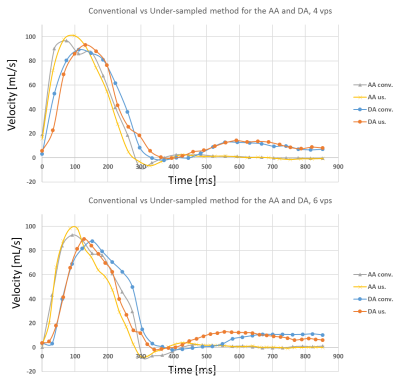
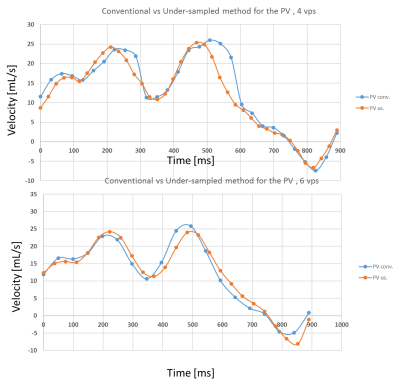
Conventional flow parameters in the pulmonary veins were successfully extracted, but S1 peak was not observable in all subjects. Mean aortic velocities were: 80.10±9.32cm/s and 85.06±4.56cm/s for the AAo and the DAo, respectively, for conventional PC and 83.30±11.20cm/s and 85.06±7.16cm/s for the under-sampled PC-MRI scheme. Mean PV velocities were: 23.16±5.08cm/s, 29.40±4.86cm/s, 29.66±2.87cm/s and -6.16±0.93cm/s for S1, S2, D and A respectively, for the conventional PC acquisition and 23.59±6.21cm/s, 30.42±4.86cm/s, 31.53±4.20cm/s and -8.34±2.29cm/s for the under-sampled PC scheme. Coefficient of variations (CoV) between aortic peaks test-retest were <5% and <7% for the S1, S2 and D peaks and <9% for the A peak for both methods. Comparison between both methods exhibited CoV of 2.45%, 1.64%, 3.72%, 3.05%, 4.49% and 19% for AA, DA, S1, S2, D and A respectively. Figure 3 and 4 compare the acquisition methods with 4 and 6 vps, with the higher frame rate PC method exhibiting a better peak identification, in particular for the AAo early slope.Conclusions
Both PC acquisitions showed peaks velocities values in line with the literature5–7. The results were comparable and showed a good correspondence of the conventional and high frame rate methods for both aortic and pulmonary vein flow. Importantly, the novel method provided potentially better accuracy, with higher velocity peaks for the aorta and pulmonary veins, due to the increased temporal resolution compared to the standard PC. This method can be applied to any phase-contrast method, and combined with any acceleration technique, potentially improving temporal resolution up to but not more than 2.Acknowledgements
No acknowledgement found.References
1. Untenberger M, Tan Z, Voit D, et al. Advances in real-time phase-contrast flow MRI using asymmetric radial gradient echoes. Magn Reson Med. 2016;75(5):1901-1908. doi:10.1002/mrm.25696
2. Ma LE, Markl M, Chow K, et al. Aortic 4D flow MRI in 2 minutes using compressed sensing, respiratory controlled adaptive k-space reordering, and inline reconstruction. Magn Reson Med. 2019;81(6):3675-3690. doi:10.1002/mrm.27684
3. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321-1360. doi:10.1093/ehjci/jew082
4. Heiberg E, Sjögren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of Segment--freely available software for cardiovascular image analysis. BMC Med Imaging. 2010;10:1. doi:10.1186/1471-2342-10-1
5. Oh JK, Appleton CP, Hatle LK, Nishimura RA, Seward JB, Tajik AJ. The noninvasive assessment of left ventricular diastolic function with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 1997;10(3):246-270. doi:10.1016/S0894-7317(97)70062-2
6. Hwang J-J, Lin J-M, Hsu K-L, et al. Correlation of the Flow Patterns among the Four Pulmonary Veins as Assessed by Transesophageal Echocardiography: Influence of Significant Mitral Regurgitation. Cardiology. 1999;91(4):256-263. doi:10.1159/000006920
7. Buffle E, Kramarz J, Elazar E, et al. Added value of pulmonary venous flow Doppler assessment in patients with preserved ejection fraction and its contribution to the diastolic grading paradigm. Eur Heart J – Cardiovasc Imaging. 2015;16(11):1191-1197. doi:10.1093/ehjci/jev126
Figures