2259
Reducing k-space gaps of golden angle rotated spiral MRI binned into cardiac frames applied to flow measurements of the coronary arteries1Department of Biomedical Engineering, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Velocity encoded MRI has clinical significance in the diagnosis of coronary artery disease. High spatial and temporal resolution increase the accuracy and reproducibility and preserve reliable flow pattern. By using rotated golden angle with k-t space reconstruction and retrospective cardiac gating, high quality flow CINE could be achieved with high spatial and temporal resolution during a single breath-hold. However, binning to cardiac frames may lead to large gaps in k-space. In this work, we propose a new scheme of rotated golden angle and optimized retrospective gating by shifting the binning window. Improved image quality is demonstrated with the proposed methods.
Introduction
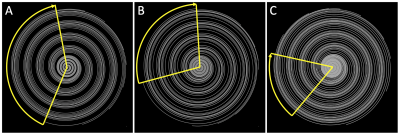
Flow velocity measurement using phase contrast (PC) MRI has been validated for clinical diagnosis of coronary artery disease1. High spatial resolution is required for high accuracy and reproducibility2, while high temporal resolution better preserves flow pattern, which is known to be affected by coronary diseases and provide information on disease state3. Clinical assessed single breath-hold approaches achieve either merely high spatial4 or high temporal3 resolution. A recently proposed method using golden angle (GA) rotated spiral sampling and k-t sparse parallel imaging reconstruction (GASSP) has been evaluated on right coronary artery (RCA) with both high temporal and spatial resolution5. However, data binning of spiral arms using retrospective cardiac gating may result in large k-space gaps in certain CINE frames that may lead to image artifacts (Figure 1A). In this study, we reset the rotation of the spiral arms to the golden angle after each ECG trigger to minimize those k-space gaps and optimize the position of the binning window to further minimize the gaps. This approach enables to reject data at the end of the acquisition for patients who cannot hold their breath for 20s, which is not possible with segmented GA approaches that are otherwise very efficient in reducing the gap size6.Methods
Proposed new scheme of rotating angle: In the traditional scheme5, each spiral arm was applied to both positive and negative flow encoding and then rotated in golden angle (i.e. 137.508°)7 for the next arm. 500 spiral arm pairs were acquired in a single breath-hold of 20s.In this work, we proposed to use a new scheme, with flow encoding direction toggled every heartbeat (using ECG detected R-wave trigger). The first arms after each R-wave was rotated by half of golden angle (i.e. 68.754°) relative to the first arm of the previous heartbeat. Within each heartbeat, consecutive arms were rotated by 83.8° so that arms of either positive of negative flow encodings are rotated by the GA. Within each heartbeat, consecutive arms were rotated by 83.8°.
Shifting the binning window to minimize gap: Data from each arm were retrospectively binned into cardiac frames for both velocity encodes according to ECG (without sliding window). Number of cardiac frames $$$n_{cf}$$$ was calculated by mean heartbeat duration over repetition time, TR = 19.2ms. The largest spiral arm gap (max-gap) of each cardiac frame and velocity encoding was determined (Figure 1). Mean-max-gap was defined as the average of all max-gaps. This process war repeated 20-times: Each time the retrospective binning window was shifted by TR/20. The shift leading to the lowest mean-max-gap was used for image reconstruction. This was performed for both the traditional and proposed angle rotation schemes. For illustration, the shift leading to the largest mean-max-gap was applied as well.
Simulation: The compare the gap size, both traditional and new schemes were computed with simulated heart rate (HR) ranging of 40 to 100 beats per minute. The simulated HR randomly changes RR interval with a normal distribution, with mean value of 60/HR (in seconds) and standard deviation of 50ms8. Computation for each HR was repeated 100 times.
In-vivo Measurements: Flow data was acquired in the popliteal artery of 4 subjects (age 26-58, 2 females) and in both right coronary artery (RCA) and left anterior descending artery (LAD) of 3 subjects (age 24, 40, and 56, 2 female) in a 3T Philips Scanner (Achieva, Philips Healthcare) using a 32-channel cardiac array. ECG signal was collected during each scan. Data with both the traditional scheme (GA-old) and the proposed scheme (GA-new) were acquired. Both were conducted with 2D gradient echo and two-sided velocity encoding in slice direction. Parameters: Slice Thickness = 8mm, field of view 350mm, and voxel size (0.8mm)2, TR/TE=19-20/2.7ms, scan time 20s. Imaging plane was planned orthogonally to target arteries on scout images.
The binned data were reconstructed with phase sensitive k-t sparse reconstruction that jointly reconstructs images from both velocity encoding.
Data analysis: Flow-encoded images were combined with geometric mean to generated flow-compensated images, which is then utilized in deblurring9 and segmentation process. Segmentation of coronary arteries was semi-automatically drawn on Medis platform with Qflow 8.1 application (Leiden, Netherlands).
RESULTS AND DISCUSSION
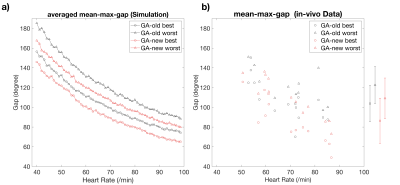
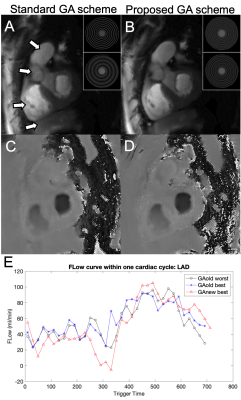
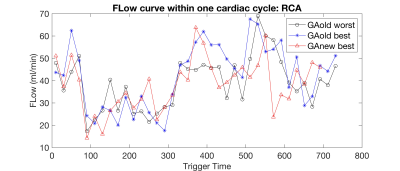
Fig 2 a) shows the decrease in mean-max-gaps could be of all simulated heart rate. Mean-max-gap of in-vivo cases demonstrated in Fig 2 b) indicates that new GA schemes significantly reduce mean-max-gap.Fig 3 a, c) demonstrate a large gap in binned data with the best shifted binning window using old scheme leading to patchy artifacts. By using new scheme, the gap is largely reduced and the image quality is greatly improved Fig 3 b, d). Flow curve shown in Fig 3 e) indicate that the flow curve with new scheme and the best shifted binning window has higher flow peak and smaller fluctuation.
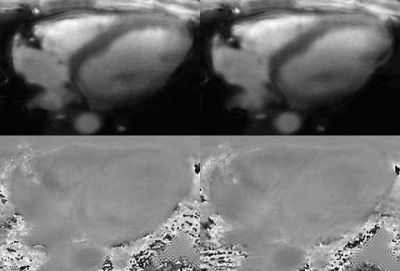
Animated Gif showing of the magnitude and through-plane velocity shown in Fig 4 demonstrate high spatial and temporal resolution and improved image quality with the new GA scheme. RCA flow curve of old and new schemes shown in Fig 5 agree well.
CONCLUSION
With the proposed new scheme of rotating angle and shifted binning window, the k-space gaps are reduced and image quality is more stable.Acknowledgements
No acknowledgement found.References
1. Ofili EO, Labovitz AJ, Kern MJ. Coronary flow velocity dynamics in normal and diseased arteries. Am J Cardiol. 1993;71:3D–9D
2. Johnson K, Sharma P, Oshinski J. Coronary artery flow measurement using navigator echo gated phase contrast magnetic resonance velocity mapping at 3.0 T. J Biomech 2008; 41: 595–602.
3. Keegan J, Raphael CE, Parker K, et al. Validation of high temporal resolution spiral phase velocity mapping of temporal patterns of left and right coronary artery blood flow against Doppler guidewire. J Cardiovasc Magn Reson 2015;17:85.
4. Brandts A, Roes SD, Doornbos J, Weiss RG, de Roos A, Stuber M, Westenberg JJM. Right coronary artery flow velocity and volume assessment with spiral K-space sampled breathhold velocity-encoded MRI at 3 tesla: Accuracy and reproducibility. J Magn Reson Imaging. 2010;31:1215–1223.
5. Zhu D, Bonanno G, Weiss RG, et al. Phase contrast coronary blood velocity mapping with both high temporal and spatial resolution using Golden Angle rotated Spiral k-t Sparse Parallel imaging (GASSP). In Proceedings of the 27th Annual Meeting of the ISMRM, Montreal, Canada, 2019:1591.
6. Han F, Zhou Z, Rapacchi S, Nguyen KL,Finn JP, Hu P. Segmented golden ratio radial reordering with variable temporal resolution for dynamic cardiac MRI. Magn Reson Med 2016;76:94–103.
7. Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging. 2007;26:68–76.
8. Nunan D. et al. A Quantitative Stematic Review of Normal Values for Short-Term Heart Rate Variability in Healthy Adults. Pace 33(11):1407-17, 2010
9. Noll DC, Pauly JM, Meyer CH, Nishimura DG, Macovski A. Deblurring for non-2D Fourier transform magnetic resonance imaging. Magn Reson Med. 1992;25:319–333.
Figures