2254
Compressed sensing reconstruction of cardiac CEST-MRI preserves accuracy, sensitivity and specificity of endogenous metabolites1University of California, Berkeley, Berkeley, CA, United States
Synopsis
While accelerated imaging via compressed sensing reconstruction has been explored in several dynamic contrast settings, incorporation of compressed sensing for accelerated image acquisition has not been fully explored in the setting of cardiac CEST. Here we test the impact of various compressed sensing methods and acceleration factors upon the accuracy of endogenous cardiac CEST in mice, specifically measures of specificity, accuracy, and sensitivity.
Introduction
Chemical exchange saturation transfer (CEST)-MRI is an emerging method for in vivo imaging of metabolite levels in intact tissue. Cardiac CEST-MRI has been used to probe creatine levels in the setting of myocardial infarction1 and obesity2,3, and identified reduced myocardial creatine CEST-contrast in failing tissues. The need to time saturation pulses and acquisition to the cardiac cycle results in significantly longer scan times than stationary organs. In pre-clinical imaging in particular, the combined need for cardiac and respiratory gating extends scan times to over an hour per spatial location. Subsequently, CEST-MRI is typically performed by acquiring fewer images along a z-spectrum and calculating CEST contrast via image subtraction instead of z-spectral fitting. While accelerated imaging via compressed sensing reconstruction has been explored in several dynamic contrast settings, incorporation of compressed sensing for accelerated image acquisition has not been fully explored in the setting of cardiac CEST. Here we test the impact of various compressed sensing methods and acceleration factors upon the accuracy of endogenous cardiac CEST in mice.Methods
Seven C57BL/6 mice were anesthetized with 1 – 3% isoflurane gas and imaged using a 7T MR scanner (PharmaScan, Bruker, Ettlingen, Germany) and a 2 × 2 surface array coil (Bruker). A custom sequence was designed such that CEST-preparation (9-11 Gaussian shaped pulses, pulse duration = 13.7 ms, pulse delay = 4.56 ms) was ECG triggered and 4 golden-angle incremented radial acquisitions were performed during diastole (TR/TE = 8 / 2.1 ms, 75 segments, matrix = 192 × 192, FOV = 25 mm × 25 mm, slice thickness = 1 mm, NA = 1). Complete spectra were acquired following peak sat power of either B1 = 0.X μT or YμT, which correspond to powers for true negative and true positive creatine CEST contrast, respectively. A reference image (saturation offset = 100ppm, B1 = 0.01μT) was acquired prior to each power setting. Spectral data sets contained 53 saturation offsets, with 6 offsets from -10 to -5 ppm at 1 ppm step size, 41 offsets from -4 to 4 ppm at 0.2 ppm step size, and 6 offsets from 5 to 10 ppm at 1 ppm step size. The fully sampled datasets were under-sampled at 2x, 3x, 4x, 5x acceleration rates prior feeding into the BART Toolbox4 for image reconstruction using six different compressed sensing methods. Nonuniform fast Fourier transform (NUFFT) reconstruction of the fully sampled data was used as a ground truth, while under-sampled data was reconstructed with ℓ1-norm, ℓ2-norm, ℓ1 wavelet transform, total variation, and locally low rank reconstructions with regularization parameter = 0.001 algorithms. The myocardium was individually segmented into six regions of interest for every frame of the spectral data set. Subsequent z-spectra were fit using the sum of 5 Lorentzian functions representing water, creatine, amide proton transfer (APT), NOE effect, and magnetization transfer (MT).Results
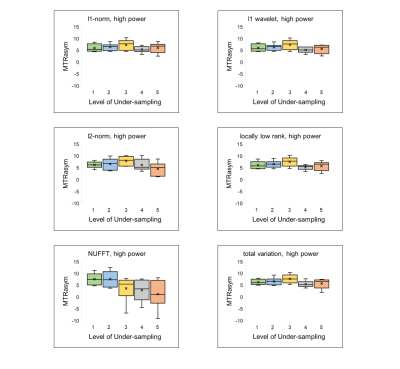
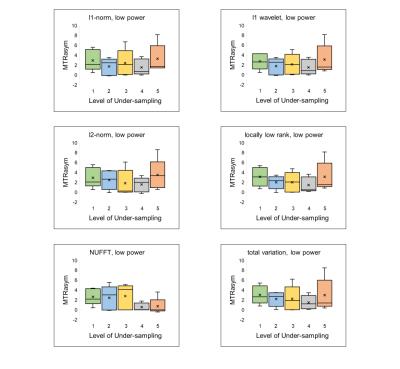
All reconstructions of CEST encoded images yielded images with poorer definition of cardiac anatomy; however, further analysis into generated CEST contrast values revealed promising detection of creatine even up to 5x acceleration. Calculated sensitivity values for l1-norm and l1 wavelet reconstructions were 100% across all acceleration factors attempted. Locally low rank and total variation techniques maintained 100% sensitivity measures for under-sampling of up to 4x of the original data. Of the six compressed sensing based reconstruction methods used in this study, l2-norm resulted in the lowest sensitivity (67%) at 5x-acceleration. Interestingly, all compressed sensing reconstruction techniques resulted in 100% specificity for acceleration factors 1-4, only dropping to 80% specificity at 5x acceleration. As expected, NUFFT reconstruction at higher acceleration factors resulted in values of MTRasym that significantly differed from reference values. Specifically, sensitivity and specificity from NUFFT images were only maintained for up to 2x under-sampling, upon which further acceleration resulted in nearly 100% error deviation in MTRasym compared to fully-sampled values. MTRasym for both high power and low power acquisitions across the six compressed sensing reconstruction methods demonstrate values of MTRasym consistent with reference values from fully-sampled NUFFT reconstruction (Figures 1,2).Discussion
The results of this study suggest that application of compressed sensing protocols to cardiac CEST data may allow for reliable detection of endogenous metabolites while reducing scan times. For measurement of myocardial creatine in particular, accuracy, sensitivity, and specificity are maintained even up to 5x acceleration. Further understanding of the extent of acceleration and robustness of reconstruction may be further explored to apply compressed sensing algorithms for analysis of spectral image sets.Acknowledgements
This study was supported by NIH 1R01HL28592 and DGE 1752814.References
1. Haris M et al., A technique for in vivo mapping of myocardial creatine kinase metabolism. Nature Medicine. 2014;20(2):209-215.
2. Diaz-Zamudio M et al., Increased pericardial fat accumulation is associated with increased intramyocardial lipid content and duration of highly active antiretroviral therapy exposure in patients infected with human immunodeficiency virus: a 3T cardiovascular magnetic resonance feasibility study. J. Cardiovasc. Magn. Reson. 2015; 17:91.
3. Pumphrey A et al., Advanced cardiac chemical exchange saturation transfer (cardioCEST) MRI for in vivo cell tracking and metabolic imaging. NMR in Biomed. 2015;29(1):74-83.
4. Uecker M et al., Berkeley Advanced Reconstruction Toolbox. Proc. Intl. Soc. Mag. Reson. Med. 23(2015) 2486.