2253
Identification of hyperelastic properties of the myocardium from CMR patient-specific 3D geometry and the Virtual Field Method.
Delphine Perie1, Mehdi Ghafari1, and Daniel Curnier2
1Mechanical Engineering, Polytechnique Montreal, Montreal, QC, Canada, 2Kinesiology, University of Montreal, Montreal, QC, Canada
1Mechanical Engineering, Polytechnique Montreal, Montreal, QC, Canada, 2Kinesiology, University of Montreal, Montreal, QC, Canada
Synopsis
Due to the complexity of the heart’s geometry, the characterization of the material properties of the myocardium remains challenging. Using kinematically admissible hyperboloid virtual fields and a finite element mesh of the left ventricle, we successfully solved the virtual field method equations to determine the stiffness of the myocardium from a reverse identification approach. The distribution of the elastic stiffness presented same patterns between the 3 volunteers. However, the standard risk volunteer presented higher stiffness within the mobile wall than the high risk volunteers. This approach would be an efficient tool to characterize early cardiac dysfunction.
Introduction
Due to the complexity of the heart’s geometry, the characterization of the material properties of the myocardium remains challenging. Modelling the mechanical behavior of soft tissues requires the knowledge of the structure and mechanical properties of the constitutive elements [1,2]. Finite elements models of the left ventricle, associated to reverse identification methods are memory and time consuming. The objective of this study was to calculate material properties of the left ventricle over the cardiac cycle using a reverse identification method based on the 3D virtual field method [3,4] and full-field data from cine-CMR.Methods
The data used in this study comes from the PETALE study [5] in which the leukemia survivors were divided into 3 groups according to their exposition risk to doxorubicin: standard risk (SR), high risk (HR) and high-risk group who received dexrazoxane, a cardioprotective agent (HRdex). The CMR acquisitions were performed on a Siemens Skyra 3T MR system using a 18-channel phased array body matrix coil and included an ECG-gated cine TruFISP sequence (14 slices in short axis and 5 slices in long axis, slice thickness 8mm, repetition time 34.6ms, effective echo time 1.2ms, flip angle 38°, iPAT factor 3, matrix 208x210 and in-plane pixel size 1.25x1.25 mm). The data points of the epicardial and endocardial left ventricle surfaces were semi-automatically extracted using CIM (v8.1, University of Auckland, [6]). These data points were imported in the 3D environment of CATIA (Dassault Systems) to create curves for the geometrical reconstruction of the endocardial and epicardial surfaces of the left ventricle. The volume delimited by these surfaces was then meshed into 10,000 hexahedral linear elements in a prolate spheroidal coordinate system (Figure 1). The displacement field of left ventricle was calculated from the differences in data points coordinates between each phase of the cardiac cycle (Figure 2). Intraventricular pressure, estimated from electrophysiological data (arterial pressure, cardiac ouput and heart rate), was applied to the endocardial surface. The finite element mesh was then used to discretize the equations of the principle of the virtual work. Using the theory of piecewise virtual fields applied to the data, we identified two kinematically admissible hyperboloid virtual fields (Figure 3) that allowed determining two unknown parameters, the global hyperelasticity (C) and the elastic stiffness (Q11) of the left ventricular myocardium. The data from one subject from each risk group of the PETALE study were used to build the models and solve the equations of the virtual field method.Results
The equations of the virtual field method were successfully solved for each of the 3 data-sets. The elastic stiffness (Q11) of the left ventricular myocardium varied from 1 to 15 kPa over the diastole cycle. It’s distribution within the myocardium at end-diastole presented same patterns between the 3 volunteers (Figure 4). However, the SR volunteer presented higher stiffness within the mobile wall than the HRdex and HR volunteers (maximum value of 14.5, 12 and 12.5 kPa for the SR, HR, and HRdex volunteers respectively). The hyperelastic parameter C increased almost linearly up to 11, 8 and 6 kPa in the SR, HRdex and HR volunteers respectively (Figure 5).Discussion
The stiffness found using this reverse identification technique based on the 3D virtual field method is in the same range as reported data in the literature from finite element models [1,2]. The convergence analysis showed the results sensitivity to the mesh size. The results were also sensitive to the chosen virtual field. A small size of the mesh and virtual fields defined in prolate spherical systems were found more suitable for measuring the material parameters than those defined in spherical coordinates. The quasi-static hypothesis simplifies the complexity of the virtual field approach developed in 3D. Incorporating the forces of acceleration and virtual speeds instead of virtual displacements would help estimate the distribution of the left ventricular mass and quantify inertial parameters. Higher stiffness in the SR group on the mobile wall may suggest less interstitial fluid in diastole than in the HR and HRdex groups.Conclusion
The identification of the mechanical properties of the myocardium from a reverse identification technique based on the 3D virtual field method would be an efficient tool to characterize early cardiac dysfunction. The technique has the potential to be easily implemented in the software of medical imaging equipments.Acknowledgements
NSERC and Polytechnique Montreal for the financial support, researchers from the PETALE study for the opportunity to do this complementary analyses on the cancer survivors.References
1- Augenstein KF, Young AA, et al. Method and apparatus for soft tissue material parameter estimation using tissue tagged magnetic resonance imaging. J Biomech Eng. 2005; 127:148–57 2- Wang VY, Nash MP, Young AA, et al. Left Ventricular Diastolic Myocardial Stiffness and End-Diastolic Myo-fibre Stress in Human Heart Failure Using Personalized Biomechanical Analysis. J cardiovascular translational research. 2018; 11(4): 346-356. 3- Grediac M, Pierron F, Avril S. The virtual fields method for extracting constitutive parameters from full‐field measurements. a review Strain. 2006; 42(4): 233-253. 4- Avril S, Badel P, et al. Anisotropic and hyperelastic identification of in vitro human arteries from full-field optical measurements. J Biomech. 2010; 43(15): 2978–2985. 5- Marcoux S, Drouin S, Laverdiere C, et al. The PETALE study: Late adverse effects and biomarkers in childhood acute lymphoblastic leukemia survivors. Pediatr Blood Cancer 2017;64. 6- Li B, Liu Y, Cowan BR, Young AA. (2010) In-line automated tracking for four dimensional ventricular function with magnetic resonance imaging. J Am Coll Cardiol Img 3, 860-866.Figures
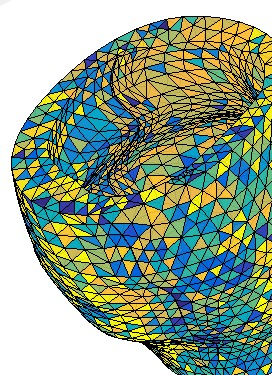
Figure 1: 3D finite element mesh of the left ventricular
myocardium.
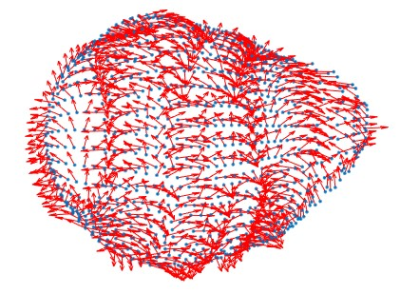
Figure 2: 3D
displacement field on the epicardium.
Figure 3: Kinematically
admissible hyperboloid virtual fields u*(1) and u*(2) with their respective
equations.
Figure 4: Distribution
of the elastic stiffness Q11 (kPa) at end-diastole on the
left ventricular myocardium for
each cancer survivor volunteer (SR, HRdex and HR).
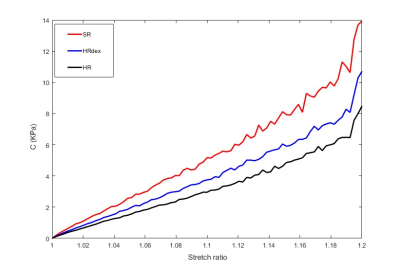
Figure 5: Hyper-elastic parameter C for
each cancer
survivor (SR, HRdex and HR) as a function of the stretch ratio.