2249
Rapid Whole-Heart 3D Cine Using a Golden Ratio Stack of Spirals Trajectory1Institute of Cardiovascular Science, UCL, London, United Kingdom, 2Great Ormond Street Hospital, London, United Kingdom, 3Institute for Biomedical Engineering, ETH Zurich, Zurich, Switzerland
Synopsis
A free-breathing three-dimensional cine imaging sequence was developed using a stack of spirals trajectory with golden angle rotations for efficient k-space sampling. Parallel imaging and compressed sensing were used in reconstruction for data acceleration. Respiratory motion artefact was avoided by binning k-space data into multiple respiratory phases. The proposed imaging protocol was tested on 10 patients and compared with a standard 2D breath-held short-axis stack. The proposed method was faster to acquire and generally able to provide correct quantitative measurements, but image quality needs to be improved and reconstruction time is too long for clinical practice.
Introduction
Free-breathing three-dimensional (3D) cine imaging is desirable because information about cardiac function and dynamic anatomy can be efficiently obtained in a single, easy to plan scan, as opposed to the complex multiplicity of protocols usually involved in cardiac MRI. However, conventional 3D cine acquisitions are very time consuming, and thus high accelerations are needed for them to be useful in the clinical environment. In addition, respiratory motion must be compensated for in order to limit the appearance of artefacts. This work aims to develop a framework for free-breathing 3D cine imaging taking advantage of the sampling efficiency afforded by spiral trajectories.Methods
Data acquisition. A free-running transverse 3D stack of spirals balanced steady state free precession (bSSFP) sequence was used to collect k-space data in free-breathing conditions. The base spiral trajectory was calculated using a publicly available numerical algorithm.1 View ordering was based on a nested loop strategy, with uniformly separated partitions in the inner loop and golden angle (∼222 °) spiral rotations in the outer loop. The following imaging parameters were used for the 3D free-breathing whole-heart acquisition (FB-WH): TR/TE = 3.42/0.95 ms, FOV = 450 x 450 x 210 mm3, matrix size = 224 x 224 x 104, voxel size = 2.0 x 2.0 x 2.0 mm3, flip angle = 60°, bandwidth = 1,590 Hz/pixel, spiral arms = 90. A total of 62,400 readouts were acquired over 3 min 33 s; enough to reconstruct 80 volumes with an approximate acceleration of 12. Gold standard data were acquired for comparison purposes using routine Cartesian 2D breath-held short-axis stacks (BH-SAX) with TR/TE = 2.34/1.17 ms, FOV = 333 x 360 x 96, voxel size = 1.5 x 1.5 x 8.0 mm3, flip angle = 68°, 40 cardiac phases. Average acquisition time for gold standard data was 4 min 48 s ± 51 s. Ten patients (age 21.2 ± 10.1 years) were recruited for this study. All imaging was performed on a Siemens Avanto 1.5T MR scanner using 12 coil elements.Data sorting. 3D FB-WH data were retrospectively gated into 20 cardiac phases and 4 respiratory phases, totalling 80 volumes. Cardiac gating information was obtained from the vector cardiogram signal. Respiratory gating information was obtained from a self-gating signal. This signal was obtained by (1) first generating head-foot projections from k-space data along the kz axis, where the spiral centes lie 2, and (2) then applying principal component analysis (PCA) and Fourier analysis to extract the respiratory component.3 The signal thus obtained was used to sort the data into respiratory bins from end-expiration to end-inspiration.4
Image reconstruction. Sensitivity maps were obtained using ESPIRiT.5 FB-WH images were then reconstructed by solving an optimization problem with sparsity constraints in the finite difference domain along the temporal dimensions (both cardiac and respiratory). Regularization parameters were selected empirically and set to 0.005 for the cardiac dimension and 0.002 for the respiratory dimension. The Berkeley Advanced Reconstruction Toolbox (BART) was used for the reconstruction.6 All reconstructions were performed in a high-performance computing (HPC) cluster node, equipped with an 18-core Intel Xeon Gold 6140 processor and 432 GB of memory. Reconstruction time per subject was 108.1 ± 2.2 min.
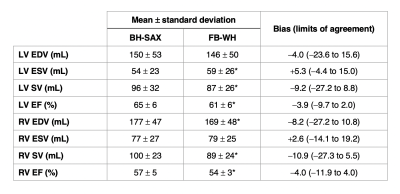
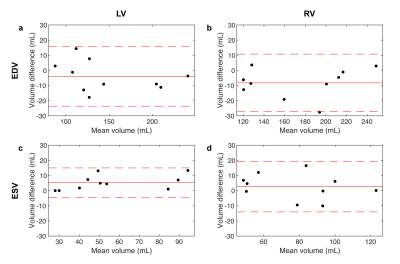
Volume quantification. FB-WH data were reformatted to a short-axis orientation in order to obtain data that is comparable to the BH-SAX gold standard. End diastolic and end systolic frames for each dataset were manually segmented by a radiologist in order to calculate clinically relevant values for left (LV) and right (RV) ventricles, namely end diastolic volumes (EDV), end systolic volumes (ESV), stroke volume (SV) and ejection fraction (EF). The resulting values were studied using t-tests and Bland-Altman analysis.
Results
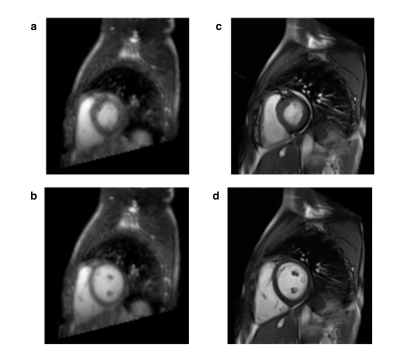
FB-WH and BH-SAX data were acquired successfully in all patients. Figure 1 shows representative images for both imaging techniques. Table 1 shows the ventricular measurements obtained from both datasets and Figure 2 displays the corresponding Bland-Altman plots.Discussion
The proposed scheme was tested on 10 patients. FB-WH acquisitions were on average 75 s shorter than BH-SAX despite providing true 3D information and did not require breath holding. However, image quality is lower. This is partly explained by the larger pixel size and higher temporal resolution. Other factors negatively affecting image quality may be the lack of fat suppression and the presence of residual respiratory motion. Nevertheless, there is quantitative agreement between both methods in clinically relevant ventricular volumes. In this regard, there is a slight underestimation of end diastolic volumes and overestimation of end systolic volumes, which is likely to be a result of temporal regularization. These biases are then reflected in derived measures such as stroke volume and ejection fraction.An important limitation of this work is the long reconstruction time, which would by itself preclude clinical implementation. Incorporating new reconstruction technologies, such as those based on deep learning, could dramatically improve the prospects in this regard.
Conclusion
In conclusion, this work presents a whole-heart imaging framework for comprehensive cardiac assessment in free-breathing conditions, with trivial planning and short acquisition time, using a stack of spirals trajectory and a compressed sensing reconstruction. Limitations remain to be addressed, namely suboptimal image quality and long reconstruction time, for the method to be clinically feasible.Acknowledgements
The authors acknowledge the use of the UCL Myriad High
Performance Computing Facility (Myriad@UCL), and associated support services,
in the completion of this work.
References
1. Pipe JG, Zwart NR. Spiral trajectory design: A flexible numerical algorithm and base analytical equations. Magn Reson Med. 2014;71(1):278-285. doi:doi:10.1002/mrm.24675
2. Kowalik GT, Steeden JA, Atkinson D, Montalt-Tordera J, Mortensen KH, Muthurangu V. Golden ratio stack of spirals for flexible angiographic imaging: Proof of concept in congenital heart disease. Magn Reson Med. 2019;81(1). doi:10.1002/mrm.27353
3. Pang J, Sharif B, Fan Z, et al. ECG and navigator-free four-dimensional whole-heart coronary MRA for simultaneous visualization of cardiac anatomy and function. Magn Reson Med. 2014;72(5):1208-1217. doi:doi:10.1002/mrm.25450
4. Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med. 2016;75(2):775-788. doi:10.1002/mrm.25665
5. Uecker M, Lai P, Murphy MJ, et al. ESPIRiT—an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med. 2014;71(3):990-1001. doi:doi:10.1002/mrm.24751
6. Uecker M, Tamir J. bart: version 0.4.04. December 2018. doi:10.5281/ZENODO.2149128
Figures