2248
Vascular Imaging of the Human Kidney with Positive Contrast via SPION and UTE
Liam Timms1, Tianyi Zhou1, Ju Qiao2, Vishala Mishra3, Gayatri Veeramani4,5, Andrew Allegretti4, Thomas Benkert6, John Kirsch2, Ravi Seethamraju7, Mukesh Harisinghani3, and Srinivas Sridhar1,8
1Physics, Northeastern University, Boston, MA, United States, 2Massachusetts General Hospital, Boston, MA, United States, 3Radiology, Massachusetts General Hospital, Boston, MA, United States, 4Nephrology, Massachusetts General Hospital, Boston, MA, United States, 5Nephrology, Brigham and Women’s Hospital, Boston, MA, United States, 6Siemens Healthcare, Erlangen, Germany, 7Siemens Healthcare, Boston, MA, United States, 8Theranano LLC, Newton, MA, United States
1Physics, Northeastern University, Boston, MA, United States, 2Massachusetts General Hospital, Boston, MA, United States, 3Radiology, Massachusetts General Hospital, Boston, MA, United States, 4Nephrology, Massachusetts General Hospital, Boston, MA, United States, 5Nephrology, Brigham and Women’s Hospital, Boston, MA, United States, 6Siemens Healthcare, Erlangen, Germany, 7Siemens Healthcare, Boston, MA, United States, 8Theranano LLC, Newton, MA, United States
Synopsis
A contrast-enhanced MRA technique using Ultra-short Time-to-Echo (UTE) pulse sequence with super-paramagnetic iron oxide nanoparticle (SPION) contrast agent is demonstrated for abdominal vascular imaging in human patients. It successfully depicts the kidney anatomy, as well as kidney cysts and kidney vascular structure in excellent clarity and detail. With manual segmentation, total kidney volume, cyst volume and renal artery diameter were estimated. This initial study in humans demonstrated the basic feasibility of abdominal QUTE-CE MRA as an alternative to Gd based CEMRA.
Introduction
An estimated 1 in 7 US adults suffer from chronic kidney disease (CKD), a progressive disease characterized by metabolic, bone and vascular complications. In order to better diagnose both the presence and severity of CKD, imaging biomarkers are uniquely positioned to capture the overall state of the pathologies (from exposure to the disease and its risk factors) while blood serum biomarkers reflect only the situation at the moment of blood draw [1]. Thus, magnetic resonance angiography (MRA) of CKD patients, holds significant clinical relevance particularly with respect to the kidney. While Contrast Enhanced MRA (CEMRA) with gadolinium (Gd) contrast agents (CAs) provides excellent sensitivity for vascular imaging, CKD is a relative-countraindication for its use (eGFR < 30 mL/min/1.73m2) due to the risks of nephrogenic systemic fibrosis (NSF) from systemic deposition of Gd [2].Therefore, a non-Gd based CEMRA technique has great potential diagnostic use in this patient population. In this study, we employ the super-paramagnetic iron oxide nanoparticle (SPION) contrast agent Ferumoxytol for abdominal CEMRA in humans with particular focus on the kidneys. By utilizing an Ultra-short Time-to-Echo (UTE) pulse sequence with Ferumoxytol we produce images with positive contrast, maximum T1-weighting and, high confinement of the CA to the vasculature due to the size of nanoparticle, enabling a new approach to CEMRA. For the first time we demonstrate this technique, which we have previously labeled quantitative UTE contrast enhanced MRA (QUTE-CE MRA) [3] can be used for angiography in human patients.
Methods
Subjects were recruited at Massachusetts General Hospital and scanned at the Martinos Center for Biomedical Imaging. Subjects were previously scheduled for 510 mg Ferumoxytol infusion as part of their regular treatment as iron therapy for anemia (FDA approved use) and consented to imaging before and after the infusion (off label use of the CA). All procedures were conducted in accordance with the Partners IRB. The majority of subjects did have known kidney disease, while one individual had advanced CKD.Pre- and post-contrast agent administration scans were acquired with a prototype 3D spiral UTE VIBE sequence at 3T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with spine and body array coils. This UTE sequence consists of a non slice-selective excitation with a stack of spirals trajectory. UTE scans were acquired in 2 regimes;
- a breath-hold scan lasting 12 seconds with TE=0.05 ms, TR=2.42 ms, FA=13 deg, resolution=2.1 mm inplane, slice thickness=2.5 mm.
- a free-breathing scan using Prospective Acquisition motion CorrEction (PACE) lasting 12 minutes with TE=0.05 ms, TR=2.91 ms, FA=13.5 deg, resolution=1.56 mm isotropic.
Results
CEMRA images were successfully acquired via the QUTE-CE technique. The technique is non-selective with respect to flow, providing contrast enhancement of the entire vasculature of the abdominal cavity (Fig. 1). From this the kidneys were manually cropped (Fig. 2).In addition to demonstrating feasibility of this abdominal imaging approach, in this initial study we segmented the kidney vasculature and anatomy, as well as kidney cysts and kidney vascular structure. This enabled measurement of kidney and cyst volume and application of the vascular modeling toolkit (VMTK, www.vmtk.org) to the vascular segmentation enabled tracking of vessel diameter along the renal artery.
Kidney artery diameters of 8 kidneys varied between 4.50 to 7.51 mm (mean +/- SD, 5.9 +/- 1.2 mm). Mean percent error for diameter measurement was 11.4% for breath-hold scans and 5.4% for free-breathing scans. Maximum percent change in renal artery diameter ranged from 5.4% to 34.9%. Using method of manual planimetry and voxel count, we measured kidney volume to be 170.8 +/- 49.0 mL (min 110.2 mL, max 233.9 mL) and cyst/kidney ratio in the range of 0.1% to 21.3%.
Discussion
These results demonstrate the feasibility of the use for UTE and Ferumoxytol as a vascular imaging technique in humans. The use of T1 weighted imaging with Ferumoxytol has been suggested for MRA in recent years [4], however with traditional sequences T1 weighting is not maximized and the strong T2 & T2* relaxation properties of Ferumoxytol lead to a complex signal dependence on concentration. Our use of a UTE sequence minimizes these effects while maximizing T1 weighting. As the technique is not selective for a particular anatomical feature, many other organs can be imaged and analyzed in this way.In previous work [3] we demonstrated the potential value of our technique, QUTE-CE MRA, for MRA but also quantification of the vasculature (CBV). Further work is ongoing in utilizing this approach for direct quantification of renal blood volume (RBV).
Conclusion
The basic feasibility of abdominal QUTE-CE MRA as an alternative to Gd based CEMRA has been demonstrated by these initial results in humans. Further, demonstration of diagnostic applicability of the potential biomarkers measured via this technique would necessitate a larger cohort. Yet, for pathologies like CKD which currenctly lacks robust imaging biomarkers, the technique may have a high degree of potential for direct application in a large patient population.Acknowledgements
This work was supported by HHS 1R41DA043974-01 awarded to Theranano LLCReferences
- Laissy, J., Trillaud, H. & Douek, P. MR angiography: noninvasive vascular imaging of the abdomen. Abdom Imaging. 2002;27(5): 488-506.
- Perazella MA. Advanced kidney disease, gadolinium and nephrogenic systemic fibrosis: the perfect storm. Curr Opin Nephrol Hypertens. 2009;18(6):519-25.
- Gharagouzloo C., Timms L., Qiao J., Zihang F., Nneji J., Pandja A., Kulkarni P., van de Ven A., Ferris C., Sridhar S. Quantitative vascular neuroimaging of the rat brain using superparamagnetic nanoparticles: new insights on vascular organization and brain function. NeuroImage. 2017;164:24–33.
- Toth GB, Varallyay CG, Horvath A, Bashir MR, Choyke PL, Daldrup-Link HE, Dosa E, Finn JP,
Gahramanov S, Harisinghani M,
Macdougall I, Neuwelt A, Vasanawala SS, Ambady P, Barajas R, Cetas JS,
Ciporen J, DeLoughery TJ, Doolittle ND, Fu R, Grinstead J, Guimaraes AR,
Hamilton BE, Li X, McConnell HL, Muldoon LL, Nesbit G, Netto JP,
Petterson D, Rooney WD, Schwartz D, Szidonya L,
Neuwelt EA. Current and potential imaging
applications of ferumoxytol for magnetic resonance imaging. Kidney Int.
2017 Jul;92(1):47-66.
Figures
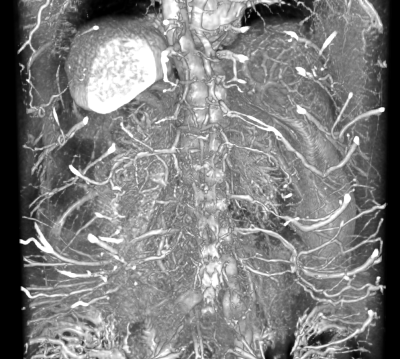
Surface rendering produced via 3D Slicer of the intensity map of the abdominal QUTECE data. View is from the posterior side such that the kidneys can be seen with close inspection.
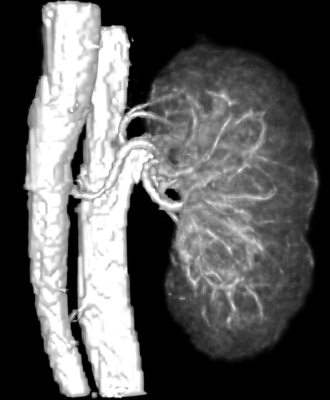
A kidney cropped from the previous image displaying the vascularture feeding into and from the organ.
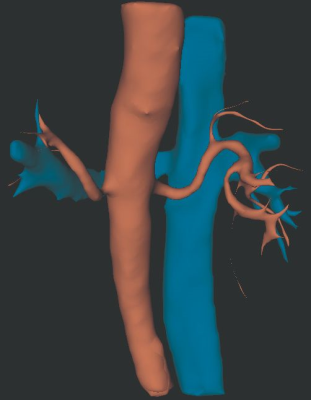
A segmentation of the arteries (red) and veins (blue) from the MRA intensity data. The renal artery can be imaged, identified and tracked.