2247
Multiplanar reconstruction of intracranial arteries: luminal morphological characterization of atherosclerosis1Radiology, University of Washington, Seattle, WA, United States, 2Surgery, University of Washington, Seattle, WA, United States, 3University of Washington, Seattle, WA, United States, 4Electrical and Computer Engineering, University of Washington, Seattle, WA, United States, 5Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Synopsis
This study aims to characterize the luminal morphology of middle cerebral arteries affected by intracranial atherosclerotic disease in a cohort of ischemic stroke patients. We used a visualization method (MOCHA) based on multiplanar reconstruction of the intracranial vasculature. We identified three distinct morphological patterns based on the effect of the atherosclerotic plaque presence on the downstream geometry. Together with the location of the plaque with respect to the middle cerebral artery branching point from the internal carotid artery, these distinct morphological patterns might be the differential factor in recurrent stroke risk stratification due to the resulting local hemodynamics.
Introduction
Intracranial atherosclerosis disease (ICAD) is a major cause of ischemic stroke worldwide with a predilection for Asian, Black and Hispanic populations, which suggests an upcoming increase in global burden as the population continues to grow in regions with those racial treats or ethnicities1,2. Furthermore, ICAD is associated with an increased risk of early recurrent ischemic stroke3. In China, stroke is the leading cause of death, with intracranial stenosis estimated to account for 46.6% of all ischemic stroke cases4. The middle cerebral arteries are the most commonly affected vessels by ICAD1.Though catheter angiography is the gold standard imaging modality to diagnose and quantify ICAD, noninvasive modalities are preferred for screening. Intracranial vessel-wall MR imaging has recently been recommended to be used in clinical practice due to its advantages for non-invasively identifying symptomatic, nonstenotic ICAD, determining the location of atherosclerotic plaque and assessing atherosclerotic plaque activity5. However, intracranial vessels tend to be tortuous, hindering precise and time-efficient plaque detection when long segments need to be analyzed. We recently developed a multiplanar reconstruction (MPR) method of the intracranial vasculature (MOCHA) that affords multiple-contrast views after straightening the vessels. Using MOCHA, we propose to characterize the downstream geometry of the middle cerebral artery (MCA) and identify the location of atherosclerotic plaques in a cohort of ischemic stroke patients.
Methods
A multi-contrast weighted 3D image visualization method for intracranial arteries was used to process the intracranial MR images. 3D TOF, pre- and post-contrast T1 weighted images were firstly registered using mutual information as the similarity metric. Then, using the TOF images, arteries were traced using a semi-automated tracing method for centerline generation6 from the intracranial internal carotid (ICA) to M1 and M2 segments of the MCA (Figure 1). Lastly, multiplanar reformation7 was performed along the artery centerline in all contrast weighted images to generate views where arteries are stretched in straight pipes.The baseline scan of ischemic stroke patients participating in the Rosuvastatin Treatment for Symptomatic Middle Cerebral Artery Stenosis Based on High-resolution Magnetic Resonance Imaging (REALM) study (NCT02041117) was used. All patients had imaging and clinically established M1 stenosis and those included in this study were followed up for a period of up to two years.
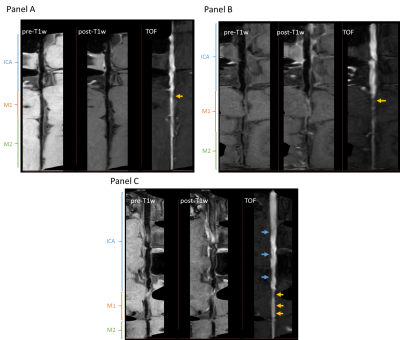
Intracranial atherosclerotic plaques were defined on the MPR images following the guidelines proposed by our group to identify definite plaque presence8. The luminal morphology was classified into three types based on their effect on the downstream geometry, as seen on the MPR images (Figure 2). Type A, tapered, is characterized by a progressive decrease in luminal diameter along the MCA length from its branching off the ICA (Panel A). In Type B, sudden, a focal plaque leads to an abrupt decrease in luminal diameter of the MCA, that persists along its length (Panel B). In Type C, sinuous, the luminal diameter follows a wavy pattern due to diffused atherosclerosis (Panel C). The location of the plaque with respect to the MCA branching from the intracranial ICA was also recorded.
Results
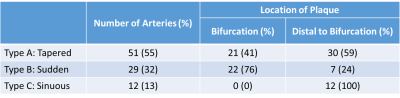
Ninety-five patients were included in the study. There were three patients without TOF images; therefore, we evaluated 92 cases (Table 1). We found that 55% of ICAD followed a tapered morphology with the plaque located distally of the ICA bifurcation into the M1 in 59% of these cases; whereas the sudden decrease in diameter was found in 32% of cases, and it was caused by the presence of plaque at the bifurcation in 76% of them. Finally, a sinuous morphology was identified in 13% of cases.Discussion
ICAD patients may share a risk of stroke due to diminish flow beyond the stenosis but have heterogeneous clinical outcomes. The differential factor in terms of outcomes may reside in the intracranial hemodynamics. Though collateralization may contribute to understand ICAD pathophysiology9, our findings further reveal that there are distinct morphological patterns of the diseased intracranial artery that might contribute to recurrent stroke risk stratification. We found that 47% of M1 plaques were located just after the bifurcation, which is a preferential location for atherosclerotic plaques due to the endothelial response to the low and oscillating shear force present in bifurcations10. Interestingly, this preferential location was not shared by the three types here described. Plaques in Type B, sudden, were more frequently found at the bifurcation compared to the other two types (Table 1), which might be explained by the contribution of additional geometrical factors upstream of the bifurcation (e.g., ICA tortuosity) to the local hemodynamics found after the bifurcation.Conclusion
We have shown that there are three distinct morphological patterns in ICAD that have the potential of being the differential factors in future clinical outcomes.Acknowledgements
The authors acknowledge our collaborators from Tiantan Hospital for sharing the images from the REALM study. The authors also acknowledge Jiarui Cai for the initial development of the registration algorithm here used, and NVIDIA Corporation for donating the Titan GPU used in this project.References
[1] Banarjee C, Chimowitz MI. Stroke caused by atherosclerosis of the major intracranial arteries. Circulation Research. 2017;120(3):502-513.
[2] Gorelick PB, Wong KS, Bae HJ, et al. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396-2399.
[3] Yaghi S, Rostanski SK, Boehme AK, et al. Imaging parameters and recurrent cerbrovascular events in patients with minor stroke or transient ischemic attack. JAMA Neurology. 2016;73:572-578.
[4] Wang Y, Zhao X, Liu L, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. 2014;45:663-669.
[5] Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the Americal Society of Neuroradiology. Americal Journal of Neuroradiology. 2017;38(2):218-229.
[6] Chen L, Mossa-Basha M, Balu N, et al. Development of a quantitative intracranial vascular features extraction tool on 3D MRA using semiautomated open-curve active contour vessel tracing. Magnetic Resonance in Medicine. 2018;79:3229–3238.
[7] Kanitsar A, Fleischmann D, Wegenkittl R, et al. CPR – curved planar reformation. IEEE Visualization. 2002:7554916.
[8] Mossa-Basha M, Watase H, Sun J, et al. Inter-rater and scan-rescan reproducibility of the detection of intracranial atherosclerosis on contrast-enhanced 3D vessel wall MRI. British Journal of Radiology. 2019;92:20180973.
[9] Liebeskind DS, Cotsonis GA, Saver JL, et al. Collateral circulation in symptomatic intracranial atherosclerosis. Journal of Cerebral Blood Flow & Metabolism. 2011;31:1293-1301.
[10] Malek AM, Alper SL, and Izumo S. Hemodynamic Shear Stress and Its Role in Atherosclerosis. JAMA. 1999;282(21):2035-2042.
Figures
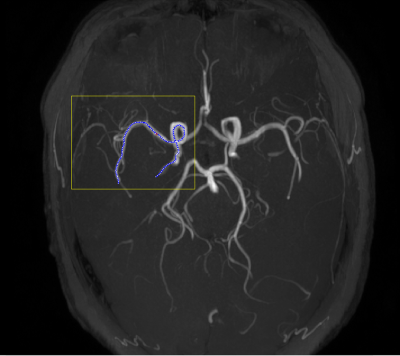
Figure 1. Maximum intensity projection (MIP) of intracranial artery disease image.The yellow box indicates the intracranial section considered in the analysis, whereas the blue dotted line indicates the traced centerline of the segment analyzed, which includes the intracranial internal carotid artery, and the M1 and M2 middle cerebral artery segments.