2246
The effect of a dynamic inversion time in high-resolution isotropic 3D dark-blood LGE without additional magnetization preparation1Department of Radiology & Nuclear Medicine, Maastricht University Medical Centre, Maastricht, Netherlands, 2CARIM School of Cardiovascular Diseases, Maastricht University, Maastricht, Netherlands, 3School of Biomedical Engineering and Imaging Sciences, King’s College London, London, United Kingdom, 4Department of Cardiology, Antwerp University Hospital, Antwerp, Belgium, 5Philips Healthcare, Best, Netherlands, 6Department of Cardiology, Maastricht University Medical Centre, Maastricht, Netherlands
Synopsis
Conventional 2D LGE often suffers from poor scar-to-blood contrast and limited spatial resolution. While 3D methods are known for their superior spatial resolution, our earlier proposed dark-blood LGE without additional magnetization preparation enables improved scar-to-blood contrast. In the present study we sought to assess the effect of a slowly increasing, dynamic inversion time on left-ventricular blood-pool suppression in a high-resolution isotropic 3D dark-blood LGE acquisition, and compare it against a conventional, fixed inversion time. Our findings show that such a dynamic inversion time significantly improves blood-pool suppression and thereby maximizes the dark-blood contrast mechanism for improved detection of myocardial scarring.
Background
Although late gadolinium enhancement (LGE) magnetic resonance imaging (MRI) is the non-invasive reference standard for myocardial tissue characterization for two decades now, LGE MRI has its clear limitations. The spatial resolution that can be achieved using 2D LGE is mainly limited by the available signal. As signal acquisition is routinely started when the healthy myocardium ‘recovered’ to the point where it has nearly no longitudinal magnetization (at the zero crossing), the resulting 2D LGE images may suffer from poor signal-to-noise ratio. In contrast to 2D MRI, 3D methods benefit from the intrinsically higher signal-to-noise ratios since the whole 3D volume is excited rather than a single 2D slice. 3D MRI is therefore able to achieve higher in-plane spatial resolution and thinner image slices with comparable signal-to-noise ratios as in 2D MRI. Additionally, when acquiring in isotropic resolution, 3D acquisitions enable the reconstruction of every desired cardiac view instead of being limited to the standard views.Another important limitation of conventional LGE is the bright signal of the blood-pool, hindering the identification of the exact border between the blood-pool and areas of scar tissue. The apparent volume of scar tissue can be significantly reduced, or even completely obscured. Recently, however, a novel and readily available dark-blood LGE method without using additional magnetization preparation was introduced that increases scar-to-blood contrast by suppressing the blood-pool signal, leading to improved detection of subendocardial scar patterns 1,2. Although the combination of 3D LGE imaging and dark-blood contrast would provide clear advantages, there is an important limitation hindering clinical implementation. As the injected contrast agent is continuously being cleared by the kidneys, the contrast agent concentration of the blood-pool slowly decreases leading to a continuously changing inversion time (TI). In order to accurate null the blood-pool during the entire 3D scan duration and maximize the dark-blood effect, a slowly increasing TI is thus required rather than a static one. We therefore translated the dark-blood LGE approach without additional magnetization preparation to 3D and implemented a dynamic TI that increases over time to compensate for contrast agent washout. In this study, we sought to evaluate the effect of this dynamic TI on blood-pool suppression in 3D dark-blood LGE and compare it with the conventional, fixed TI.
Methods
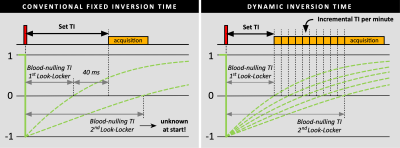
A total of 20 consecutive patients were enrolled and scheduled on a 1.5T MR system (Ingenia, Philips Healthcare). Ten minutes after intravenous injection of 0.2 mmol/kg gadobutrol, a ECG triggered respiratory navigated 3D whole-heart spoiled gradient-echo PSIR dark-blood LGE sequence without additional magnetization preparation was performed (TE: 3 ms, TR: 6.5 ms, flip angle: 25°, acquired/reconstructed resolution: 1.6/0.8 mm isotropic). For all patients, a Look-Locker scan was performed both before and after the 3D acquisition for obtaining the left-ventricular blood-pool nulling TI. In the first ten patients, a static TI was used where 40 ms were added to the blood-nulling TI obtained from the first Look-Locker to compensate for the contrast washout during the scan duration (figure 1). By using the TI from the second Look-Locker scan (after the 3D acquisition) and knowing the time between both Look-Locker scans, the average increase per min for the blood-pool nulling TI was calculated. In the second ten patients, a dynamic TI was used where the TI from the first Look-Locker scan (before the 3D acquisition) was set as initial value, and automatically increased every minute using the increment value found in the first group. Although the PSIR images are routinely used for clinical decision making, the modulus images were analysed for the degree of blood-pool suppression. Regions of interest (ROIs) were drawn in the left-ventricular blood-pool using MATLAB. Within these ROIs, the mean signal intensity of the blood-pool was calculated as a measure for blood-pool suppression, while the standard deviation was calculated as a measure for blood-pool suppression consistency. For both measures, the groups with the fixed TI and dynamic TI were compared using the independent-samples Student’s-t-test.Results
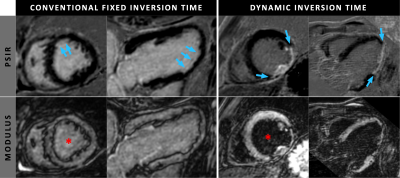
Complete data sets were successfully acquired in all 20 subjects. The results of the two Look-Locker scans in the first group showed an average increase of 2.39±0.52 ms per minute for the blood-nulling TI, which was used as incremental value for the dynamic TI in the second group. When comparing the blood-pool signal intensities, the second group with dynamic TI showed a significantly lower blood-pool intensity compared to the first group with the static TI (p=0.028, figure 2 & 3). On average, a decrease of 62.4% in blood-pool intensity was observed when using a dynamic TI rather than a static one. When considering consistency in blood-pool suppression, the group with the dynamic TI showed a significant lower spread in signal intensities throughout the blood-pool (p=0.046).Discussion
This study shows that using a dynamic TI during high-resolution isotropic 3D whole-heart acquisitions leads to improved and more consistent blood-pool suppression. This accurate suppression during the entire acquisition maximizes the proven dark-blood contrast without additional magnetization preparation, and thereby leading to improved detection and assessment of thin subendocardial scarring. By implementing this superior dark-blood contrast in a high-resolution isotropic 3D whole-heart acquisition, a novel diagnostic tool is created for excellent and detailed assessment of myocardial scar in every desired cardiac view.Acknowledgements
The authors would like to thank the radiographers and administration team of the Maastricht University Medical Centre for their cooperation and assistance during the imaging and administration processes.References
[1] Holtackers RJ, Chiribiri A, Schneider T, Higgins DM, Botnar RM. Dark-blood late gadolinium enhancement without additional magnetization preparation. J Cardiovasc Magn Reson. 2017;19(1):64
[2] Holtackers RJ, Van De Heyning CM, Nazir MS, et al. Clinical value of dark-blood late gadolinium enhancement cardiovascular magnetic resonance without additional magnetization preparation. J Cardiovasc Magn Reson. 2019;21(1):44
Figures

Multiple cardiac views of a patient acquired with the proposed high-resolution isotropic 3D whole-heart dark-blood LGE without additional magnetization preparation. Note that all cardiac views are reconstructed from the same 3D dataset that was acquired in the transversal plane. The high isotropic resolution allow for accurate visualization and assessment of both the left- and right-ventricle. Additionally, the papillary muscles can be analyzed in great detail using multiple views