2244
Magnetic Nanoparticle Sensor for Longitudinal Biomarker Monitoring1Health Sciences & Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 2Koch Institute For Integrative Cancer Research, Cambridge, MA, United States, 3Materials Science & Engineering, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Continuous biochemical monitoring remains a critical challenge in the biosensing community as longevity, biocompatibility, and signal transduction limit multi-month implantation. We propose a novel design and evaluation of the sensing capability of a magnetic nanoparticle dosimeter assay for in vivo implementation, readable by magnetic relaxation. This local diagnostic device offers continuous sentinel evaluation and longitudinal tracking of critical biomarkers found to correlate with cardiovascular disease states. The current design exhibits an order of magnitude improvement in performance compared to prior studies, translating into a predicted 29 week lifetime.
Introduction
Harnessing the diversity of sensing platforms for applications in complex biological environments has been a challenge in the field of biosensing. Electrochemical, optical, and fluorescence systems have been proposed, most with limitations in biocompatibility, sensitivity, and deep-tissue signal transduction from opaque media, to longevity under non-laboratory conditions. We have developed an implantable diagnostic device that addresses these limitations for the continuous measurement of biomarkers using a nanoparticle aggregation assay (Magnetic Relaxation Switches, MRSw).1,2 Exposure to target induces a switch from a dispersed to an assembled state with a corresponding change in magnetic relaxation properties allowing for robust contrast. Current applications focus on diagnostics in chronic and transient cardiac disease models with low sampled concentrations and temporary elevations in biomarker presence frequently missed by serial sampling. We address assay sensing functionality, tunability, and degradation to lay the groundwork for multi-month implantation.Methods
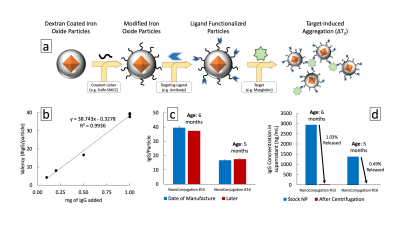
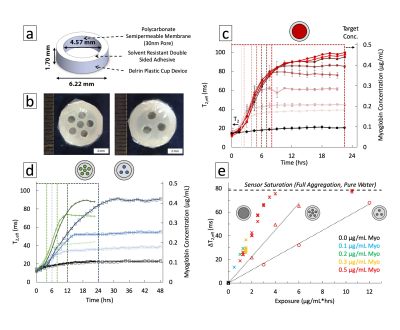
Magnetic contrast depends on the core nanomaterial while biological functionality is provided by surface ligands with an affinity toward multivalent targets (Fig. 1a). Polyclonal anti-Myoglobin was conjugated to the surface of superparamagnetic iron oxide nanoparticles with aminated dextran shells (20-50 nm). Particles were mixed with target to produce aggregation-induced magnetic contrast. Proton Relaxation measurements (transverse relaxation time, T2,eff) were acquired on a custom single-sided, inhomogeneous field, NMR relaxometer (0.43 T, 25°C, NMR MOUSE) fitted with a programmable robotic stage scanning each well of a microplate over the read region (9x9x0.3 mm).3 A CPMG pulse sequence was used: TE = 0.035 ms, 5454 echoes, 15 scans, TR = 6 s. Relaxation times were determined by a single exponential fit to echo peak intensities with a custom MATLAB script. Cylindrical Delrin diffusion devices were filled with particle colloidal suspension and sealed with a 30 nm pore polycarbonate semi-permeable membrane of varying open cross-sectional area via a double-sided adhesive (Fig. 3a). Devices were exposed to varying concentrations of target while aggregation response was read by single-sided MR every 90-120 minutes for up to 48 hrs. Degradation of particles was measured by loss of ligand from the surface and changes in MRSw performance after elevated temperature storage for up to 12 weeks.Results
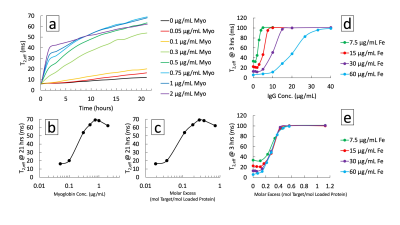
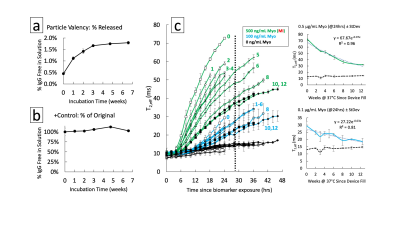
Ligand valency was tuned over an order of magnitude by varying conjugation parameters (Fig. 1b). Conjugation stability under standard storage conditions indicated a lifetime >5 months by isolating free from bound protein by centrifugation (Fig. 1c,d). A maximum of 1% of conjugated ligand was released, minimally decreasing valency. MRSw performance showed prozone agglutination behavior with quantitative exposure ranging from 50-2000 ng/mL of target and sufficient contrast in <2 hrs (Fig. 2a-c). Saturation response was shown to be tunable by varying particle iron concentration from 7.5-60 μg/mL. Comparing response curves showed that molar excess of target to ligand is a unifying saturation metric (Fig. 2d,e). Device dosimeter performance was validated for the Myoglobin model. Unmodified devices were compared to reduced cross-section devices (3 & 6 holes) in MRSw performance to temporary biomarker elevations. Saturation was tuned from 4 to >12 μg/mL*hrs corresponding with 8 to >24 hrs of biomarker elevation at 500 ng/mL (Fig. 3c-e). 4 hr equilibration and signal stability post-exposure for >12 hrs was also observed. Biochemical and aggregation performance degradation parameters were evaluated. Over 6.5 weeks of elevated temperature storage, <2% of the conjugated antibody was unbound (Fig. 4a,b). Control antibody did not show appreciable drift in binding performance as measured by ELISA. MRSw longitudinal performance on exposure to target after 0-12 weeks of incubation showed a monotonically decreasing response rate (Fig. 4c). Exponential fit to decay curve at 24 hrs predicts a 29 week “switch” (on/off) sensor lifetime for an infarction condition (500 ng/mL Myo) (Fig. 4c, Top Inset).Discussion
The MRSw system can be derivatized for a cardiac biomarker with usable sensitivity and saturation dosimeter response corresponding to elevations expected in a Myocardial Infarction (MI). Device performance can be tuned by matching sensor characteristics (membrane cross-sectional area, particle size, ligand affinity and valency, etc.) with the physiological range of target sensitivity and saturation desired. By tuning surface chemistry and bioconjugation strategies we have enhanced the performance of the switch-based system by an order of magnitude over prior studies. Assay usable lifetime and nanostructure degradation are key parameters in multi-month applications. We have shown the limiting factor is not the loss of ligand from the surface of the particles but rather the loss of binding activity as seen in elevated temperature experiments.Conclusion
This implant has the potential to broaden diagnostics in personalized medicine by addressing the hurdles of longevity and robust sensor signal stability in complex environments. Translation of single-sided relaxometry results to deep tissue measurement of biosensor output is made possible by MR localization and the large contrast produced by target-induced aggregation. In situ diagnostics offer sentineling of critical biomarkers, providing a deeper understanding of the local biology in heterogeneous systems. Continuous monitoring provides valuable timescale data to clinicians giving them insight into patients’ lab results for early intervention and data collection throughout treatment. This implant has the capacity to bridge the gap between lab and clinical-grade sensors, significantly improving preventative and interventional medicine by leveraging the robust and tunable nature of the MRSw assay.Acknowledgements
Funding provided by the National Science Foundation Graduate Research Fellowship Program. This work made use of the MRSEC Shared Experimental Facilities at MIT, supported by the National Science Foundation under award number DMR-14-19807. The authors also thank the Hammond Lab for their assistance with the project.References
1. Daniel KD, Kim GY, Vassiliou CC, et al. Implantable diagnostic device for cancer monitoring. Biosens Bioelectron. 2009;24:3252-3257
2. Ling Y, Pong T, Vassiliou CC, Huang PL, Cima MJ. Implantable magnetic relaxation sensors measure cumulative exposure to cardiac biomarkers. Nat Biotechnol. 2011;29(3):273-277
3. Blumich B, Blumler P, Eidmann G, et al. The NMR-MOUSE: Construction, Excitation, and Applications. Magn Reson Imaging. 1998;16(98):479-484
Figures