2241
16-fold accelerated real-time free-breathing cardiac cine MRI in patients with a cardiac implantable electronic device1Radiology, Northwestern University, Chicago, IL, United States, 2Translational and Molecular Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Division of Cardiology, Internal Medicine, Northwestern University, Chicago, IL, United States
Synopsis
CMR measurements in CIED patients are critical but challenging due to susceptibility artifacts. We described the development of highly accelerated, real-time, free-breathing cine pulse sequence using compressed sensing with variable density “lattice-like” k-space sampling scheme. Comprehensive comparison was done to estimate performance against clinical standard breath-hold cine pulse sequence.
Introduction
Recent evidence suggest that it is possible to perform cardiovascular MR (CMR) with manageable risk in patients with a cardiac implantable electronic device (CIED).1,2 Image artifacts induced by CIED and high burden of arrhythmia and dyspnea, however, pose technical challenges for successful CMR in CIED patients. In response, we sought to develop a 16-fold accelerated, real-time, free-breathing cine pulse sequence using gradient echo readout and compressed sensing and evaluate its performance against retrospective ECG-gated cine pulse sequence using gradient echo readout in CIED patients.Materials and Methods
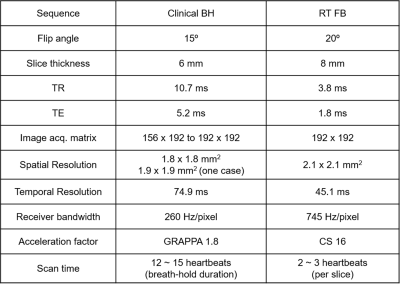
Patients 13 consecutive patients (age 59 ± 12 years; 9 men and 4 women) with a CIED undergoing a clinical CMR were recruited. MRI experiment CMR was performed on a whole-body 1.5 T MRI scanner (Avanto, Siemens). Relevant imaging parameters for the clinical standard breath-hold cine MRI and our real-time cine MRI acquisitions are summarized in Table 1. We used a 16-fold accelerated “lattice-like” Cartesian k-space sampling pattern with variable density along ky to achieve a good balance between inherence2 and self-calibration of coil sensitivities by using the time-averaged images and processing it further, as previously described.3 For the clinical standard acquisition, despite having a poor temporal resolution = 74.9 ms, images were interpolated along time to produce 25 cardiac frames per heartbeat. Image reconstruction Figure 1 shows our CS reconstruction pipeline implemented on a GPU workstation (16 GB NVIDIA Tesla V100; 256 GB CPU RAM). To accelerate the image reconstruction, we applied software coil compression using PCA4 and used the gpuArray function in MATLAB. After self-calibration of coil sensitivities, zero-filled images were dealiased using iterative CS5 (30 iterations, conjugate gradient with backtracking line search, temporal total variation and temporal PCA as two orthogonal sparsifying transforms). Image analysis For visual assessment, two experienced cardiovascular imaging attendings graded clinical standard and our real-time cine images in a randomized fashion using a 5-point Likert scale for the following 4 categories: conspicuity of myocardial edge definition (1: nondiagnostic to 5: excellent), temporal fidelity (1: nondiagnostic to 5: excellent), artifact level (1: nondiagnostic to 5: minimal) and noise level (1: nondiagnostic to 5: minimal). A score of 3 was defined as clinically acceptable. One clinical research fellow analyzed the cine images using the QMass7.6 software (Medis, Leiden, Netherlands), in order to compute end diastolic volume (EDV), end systolic volume (ESV), stroke volume (SV) and ejection fraction (EF). Statistical analysis A Wilcoxon rank-sum test was used to compare visual score (myocardial edge definition, temporal fidelity, artifacts, and noise) between breath-hold cine and free-breathing cine. P values of less than 0.05 were considered statistically significant.Results
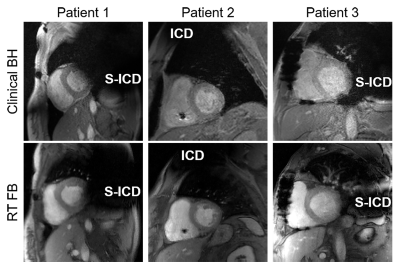
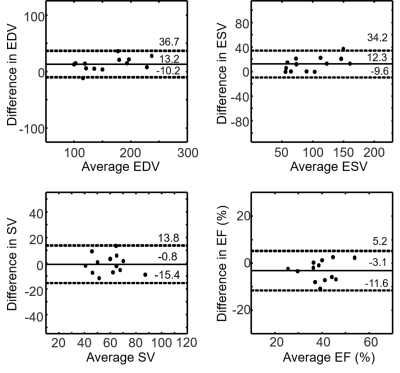
The CS image reconstruction time on average was 52 sec per slice with 44 frames. Figure 2 compares images obtained by clinical standard cine and our real-time cine in three representative CIED patients. As shown in Fig. 2, our real-time images produced similar image quality. Table 2 shows the median scores of myocardial edge definition, temporal fidelity, artifacts, and noise. While temporal fidelity and noise scores were significantly different, they were both above the clinically acceptable cutpoint (3.0). Figure 3 shows Bland-Altman analysis illustrating agreement in cardiac functional parameters between clinical standard cine and real-time cine. For EDV, the mean difference was 13.2 mL (8.3% of mean) and limits of agreement (LOA) was 36.7 mL (23.2% of mean). For ESV, the mean difference was 12.3 mL (12.6% of mean) and LOA was 34.2 mL (35.1% of mean). For SV, the mean difference was -0.8 mL (1.3% of mean) and LOA was 13.8 mL (2.3% of mean). For EF, the mean difference was -3.1% (7.9% of mean) and LOA was 5.2% (13.2% of mean).Discussion
The proposed 16-fold accelerated real-time free-breathing cine MRI with CS reconstruction is capable of producing diagnostically acceptable image quality and reliably quantifying left ventricular EF in CIED patients. A future study is warranted to evaluate the performance of said acquisition in a diverse cohort of CIED patients.Acknowledgements
No acknowledgement found.References
1. Maass AH, Hemels MEW, Allaart CP. Magnetic resonance imaging in patients with cardiac implantable electronic devices. Neth Heart J. 2018;26:584–590.
2. Hong K, Collins JD, Freed BH, et al. Accelerated Wideband Myocardial Perfusion Pulse Sequence with Compressed Sensing Reconstruction for Myocardial Blood Flow Quantification in Patients with a Cardiac Implantable Electronic Device. Radiology: Cardiothoracic Imaging (In press)
3. Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med. 2000;43:682–690.
4. Huang F, Vijayakumar S, Li Y, Hertel S, Duensing GR. A software channel compression technique for faster reconstruction with many channels. Magn Reson Imaging. 2008;26:133-141.
5. Feng L, Srichai MB, Lim RP, et al. Highly accelerated real-time cardiac cine MRI using k-t SPARSE-SENSE. Magn Reson Med. 2013;70:64–74.
Figures