2237
Accelerated Phase Contrast MRI Reconstruction with Deep U-NET Convolutional Neural Networks1ECE, University of Louisville, Louisville, KY, United States, 2Cardiovascular Medicine, University of Louisville, Louisville, KY, United States
Synopsis
We propose a framework for accelerated reconstruction of 2D phase contrast MRI from undersampled K space by using deep convolutional neural networks. The reconstruction problem is considered as a de-aliasing problem in complex spatial domain. A U-net architecture was trained and tested on 4D flow MRI data in 10 patients with aortic stenosis and 4 healthy volunteers. The reconstructed complex two channel image showed that the U-net is able to unaliase the undersampled flow images with resulting magnitude and phase difference images showing good agreement with the fully sampled magnitude and phase images.
Introduction
Phase Contrast MRI is an imaging method which can non-invasively derive hemodynamic information inside the human body. With the advent of 4D flow imaging, scan time has become a significant issue. Various methods were proposed to reduce scan time in 4D flow imaging which includes compressive sensing[1] , Kt SPARSE SENSE[2], KT BLAST[3] etc. However, computationally expensive methods deters the possibility of real time reconstruction. Recently, deep CNN architecture’s ability to identify intricate features from data has shown great promise in medical image reconstruction [4] within seconds. In proposed method, we chose a U-net based architecture for PC MRI reconstruction from undersampled K space.Method
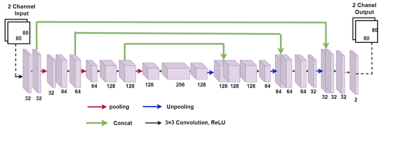
From fully sampled k-space data undersampling in K space was performed in the phase-encode direction based on a probability density function which ensures maximum rate of sampling in low frequency region. Figure 1 shows network architecture of proposed U net. The network takes an 80 × 80 two channeled image as input where the channels store real and imaginary part of the folded complex image. The U-net has in total 7 convolutional layer in contraction and 7 convolution layer in expansion. Pixel wise mean squared error between the output and labelled image was considered as loss function. 4D flow data of blood flow through the aortic valve was collected with Cartesian readout in 10 patients and 4 healthy volunteers on a 1.5T Phillips Achieva scanner. The scan parameters for patient data were, TE= 3ms, TR= 14ms, number of frames = 15, matrix size=80×80×10, Field of View=200×200×50 (mm), slice thickness= 5mm, number of slices=10. Respiratory gating with navigators was used. The FOV included 1-2 slices proximal to the aortic valve with the remaining 8-9 slices distal to the valve. Fully sampled K space data were acquired on the scanner and down sampled offline with different sampling rates. Each time frame from every slice was taken as separate training example. Despite this, the training dataset was relatively small containing only 2100 images. Therefore, the dataset was augmented by rotating each image between [0, 2π] in 10 degree increments, thus creating a dataset of 73,500 images. We split the dataset into training and testing sets via 7 fold cross validation on 14 subjects. A small batch size of 32 was used to train the network. Network weights were initialized using normal distribution with standard deviation of 0.01. RMSpropoptimizer was used to minimize the loss function with a learning rate of 0.001 and epoch number of 500. We used Nvidia’s GeForce GTX 1050 Ti GPU and training took 1 day. The experiments in this study were performed using Keras with Tensorflow whose back end is Python 2.7.Results
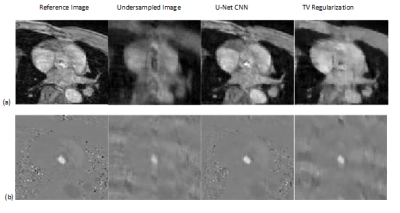
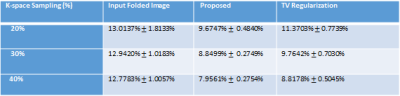
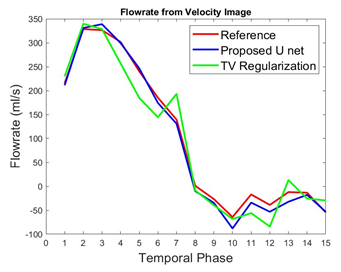
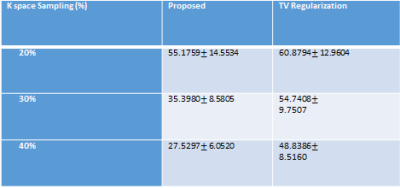
To validate the proposed method, normalized mean square error (NMSE) was calculated as reconstruction error. We compared the NMSE error with a state of the art TV regularization method [5] where spatial TV minimization was performed in split bregman iterative process. Blood Flow rate in the velocity mapped image was measured by calculating mean velocity in the flow region multiplied by vessel area. Accuracy of flow rate was measured by calculating RMSE between flow rate calculated from reconstructed velocity mapped image and fully sampled velocity mapped image. Figure 2 shows reconstruction results of 1 image slice in patient data with 30% undersampling. Figure-2(a) shows magnitude image for Reference fully sampled image, folded undersampled image, reconstructed image by proposed method and reconstructed image by TV regularization. Figure 2(b) shows the corresponding phase difference images. It demonstrates that reconstructed magnitude and phase image by the proposed U-net can restore structural information and fine details of original image discarded by the undersampling. Table 1 shows average NMSE for different undersampling rate. From average error results it is evident that the proposed network performs better than TV regularization for 3 different undersampling rates. Figure 3 shows blood flow with time in a slice close to aortic valve in one patient. The figure shows that flow rate from reconstructed phase image from 30% undersampling with the proposed method is almost identical to reference phase image flow rate. Table II shows average RMSE in the measured flow waveform for different K space sampling rates across all subjects and all slices from7 fold cross validation.Discussion:
We have presented a first application of deep learning to 2D single slice-based reconstruction of 4D flow MRI from undersampled data. We have shown that the proposed network can learn the complex domain features of PC MRI and is able to reconstruct the data with good performance.Acknowledgements
Support from the National Institutes of Health (grant 1R21-HL132263) is gratefully acknowledged.References
[1] Z.Chen, X. Zhang, C. Shi, S. Su, Z. Fan, JX. Ji, G. Xie, X. Liu., "Accelerated 3D Coronary Vessel Wall MR Imaging Based on Compressed Sensing with a Block-Weighted Total Variation Regularization", Applied Magnetic Resonance, vol. 48, no. 4, pp. 361-378, 2017. Available: 10.1007/s00723-017-0866-0
[2] D. Kim, H. Dyvorne, R. Otazo, L. Feng, D. Sodickson and V. Lee, "Accelerated phase-contrast cine MRI using k-t SPARSE-SENSE", Magnetic Resonance in Medicine, vol. 67, no. 4, pp. 1054-1064, 2011. Available: 10.1002/mrm.23088.
[3] M. Carlsson et al., "Quantification and visualization of cardiovascular 4D velocity mapping accelerated with parallel imaging or k-t BLAST: head to head comparison and validation at 1.5 T and 3 T", Journal of Cardiovascular Magnetic Resonance, vol. 13, no. 1, 2011. Available: 10.1186/1532-429x-13-55
[4] C. Hyun, H. Kim, S. Lee, S. Lee and J. Seo, "Deep learning for undersampled MRI reconstruction", Physics in Medicine & Biology, vol. 63, no. 13, p. 135007, 2018. Available: 10.1088/1361-6560/aac71a.
[5] T. Goldstein and S. Osher, "The Split Bregman Method for L1-Regularized Problems", SIAM Journal on Imaging Sciences, vol. 2, no. 2, pp. 323-343, 2009. Available: 10.1137/080725891
Figures