2236
Fully automated quantification of left ventricular scar in patients with ischemic heart disease using deep learning and Gaussian mixture models1King's College London, London, United Kingdom, 2Eindhoven University of Technology, Eindhoven, Netherlands, 3Philips Healthcare, Best, Netherlands
Synopsis
Late gadolinium enhancement MRI is crucial tool for guiding the management of patients with known/suspected myocardial infarction. Currently, clinical practice relies on the visual inspection of these images but there has been extensive work on semi-automated approaches for quantifying scar. Their utility is however limited by the time-intensive manual interactions involved, including the drawing of the myocardial contours. In this work, deep learning methodology is employed to automatically achieve the previously manual steps and these segmentations are used as input to a completely unsupervised scar quantification algorithm. This allows automatic, fast and reproducible quantification of regions of scar.
Introduction
The clinical use of late Gadolinium Enhancement (LGE) cardiovascular magnetic resonance (CMR) to guide the management of patients suspected of having a myocardial infarction requires an accurate delineation of regions of scarred myocardial tissue. The reference standard for this assessment is a semi-automated approach, based on thresholding. The most reproducible of these is the full width-at-half maximum (FWHM) approach.1 This requires the drawing of endocardial and epicardial contours and further segmentation of a hyper-enhanced region of the myocardium. The algorithm will then apply a threshold based on half of the maximum intensity in the selected hyper-enhanced region in order to segment the scar. Despite the semi-automated nature of the algorithm, it is still time consuming, which limits its clinical applicability. In this work we propose a fully automated pipeline for the quantification of left ventricular scar from a 2D short-axis stack of LGE images.As opposed to the approach of Fahmy et al.2 which directly used deep learning to detect both the myocardium and scarred tissue, our approach builds on the idea of thresholding but automates the manual steps. We propose that this approach will be more robust than directly segmenting the scar, as this can be difficult due to the vast differences in the appearance, positioning, size and associated signal intensity values of the scar and the difficulty of acquiring reliable training data.
Methods
CMR scans were performed at 3T (Philips Achieva TX, Philips Medical Systems, Best, Netherlands) and the LGE images were acquired according to standard clinical protocol. Images were acquired using a magnitude inversion-recovery (IR) gradient-recalled echo sequence, the inversion time was chosen to null the signal of viable myocardium. A total of 155 patients were included in this study (130/10/15; training/validation/test). The training labels were generated semi-automatically using the FWHM approach with the cvi42 software (Circle Cardiovascular Imaging Inc., Calgary, Alberta, Canada) by an experienced operator (A.V, S.F).Our automated processing pipeline is similar to that which we have recently proposed for the analysis of stress perfusion CMR.3 The first step of this is to compute a bounding box encompassing the left ventricle and left ventricular myocardium. This cropping reduces some of variability in the data and results in more accurate segmentation. The myocardium is then segmented and the mask is then sub-segmented into small clusters of pixels, called superpixels. Finally, an automated thresholding approach is then applied in order to classify the superpixels as either scar or normal tissue.
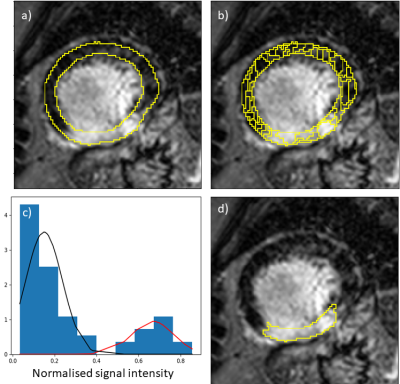
The bounding box is computed using a region-proposal network, this is a convolutional neural network (CNN) architecture which has been trained to take a proposed bounding box for the region of interest and to transform it so that it includes the LV and myocardium. The myocardium is then segmented using a U-Net4 architecture (Figure 1(a)). Once the myocardium has been segmented, a threshold is needed in order to segment the infarcted region.
The myocardium is first sub-segmented into superpixels which uses the simple linear iterative clustering (SLIC) algorithm to segment the myocardium into small coherent regions. The superpixels are computed using k-means clustering on the intensity values, this creates regions of pixels with similar intensities and thus the superpixels respect the underlying structure of the image (Figure 1(b)).
The superpixels are classified as scarred or normal myocardium by fitting a Gaussian mixture model (GMM) to the superpixel intensity values (Figure 1(c)), similar to Engblom et al.5 Based on this approach, superpixels are classified either based on the presence of two classes (both viable and scarred myocardium) or one class (all normal or all scarred myocardium). This is automatically decided by fitting both the one and two class GMM to the data and choosing the model that yields the lower Bayesian information criterion value. The superpixels are classified according to which Gaussian distribution they most likely belong to (Figure 1(d)).
The methods are evaluated by comparing the DSC between the automated and manual segmentations of the bounding box, myocardial and scar segmentations.
Results
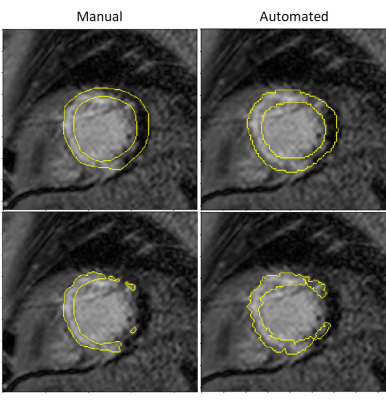
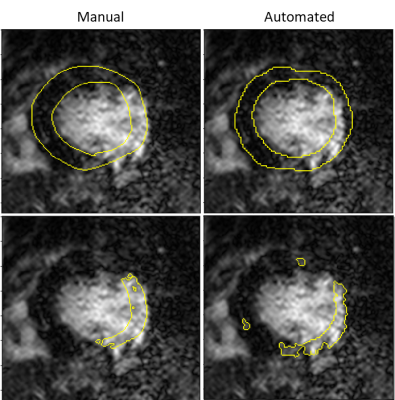
The mean (SD) Dice similarity coefficient (DSC) between the manually derived and automated bounding box was 0.91 (0.06). The mean (SD) DSC between the manual and automated myocardial segmentation was 0.79 (0.1) and the mean (SD) DSC between the semi-automated and fully automated scar segmentation was 0.52 (0.27).Examples of the myocardial and scar segmentations for representative test cases are shown in Figure 2 and Figure 3.
Discussion and conclusion
We have shown that a combined deep learning and thresholding approach to the analysis of LGE CMR images has the potential to lead to fast, automated and reproducible segmentation of the myocardium and quantification of regions of myocardial infarction. This could potentially allow routine scar quantification in the clinic and could easily be combined with other cardiac parametric mapping techniques for a full disease assessment. Though the lack of ground-truth in this case restricts our ability to investigate the true performance of the approach. Future work will include a more thorough evaluation of the methods, including an investigation of the clinical utility of the approach.Acknowledgements
The authors acknowledge financial support from the King’s College London & Imperial College London EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1); Philips Healthcare; The Alan Turing Institute under the EPSRC grant EP/N510129/1; The Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust and via the NIHR Cardiovascular MedTech Co-operative at Guy’s and St Thomas’ NHS Foundation Trust; The Centre of Excellence in Medical Engineering funded by the Wellcome Trust and EPSRC under grant number WT 088641/Z/09/Z.References
[1] Grani, C. et al. Journal of Cardiovascular Magnetic Resonance 21 (14) 2019
[2] Fahmy, AS. et al. JACC: Cardiovascular Imaging 11 (12) 2018
[3] Scannell, CM. et al. Journal of Magnetic Resonance Imaging (in press) 2019
[4] Ronneberger, O. et al. MICCAI 2015
[5] Engblom, H. et al. Journal of Cardiovascular Magnetic Resonance 18 (27) 2016
Figures