2234
Deep Learning based Semantic Segmentation of Scar Tissue in Late-Gadolinium Enhancement CMR – First Results1Department of Diagnostic and Interventional Radiology, University Hospital Würzburg, Würzburg, Germany, 2Congestive Heart Failure Center, University Hospital Würzburg, Würzburg, Germany
Synopsis
Quantitative analysis of scar tissue in late gadolinium enhancement (LGE) cardiac magnetic resonance imaging (CMR) typically requires manual or at best semi-automatic segmentation by a trained physician. To supersede this time-consuming and tedious task, a convolutional neural network with a U-Net architecture and a ResNet34 backbone was trained for semantic segmentation of scar tissue in LGE CMR. The predictions of the proposed model yielded high performance for the detection of focal scar tissue and bears thus potential for fully automated and consequently time-efficient post-processing.
Introduction
Late gadolinium enhancement (LGE) cardiac magnetic resonance imaging (CMR) represents the standard clinical tool for the evaluation of myocardial viability and the quantification of non-viable scar tissue in the case of ischemic myocardial disease. Subsequently to intravenous bolus application, injected gadolinium(Gd)-based contrast agent accumulates in non-viable scar tissue and can ultimately be detected as a focal and hyper-enhanced area within the myocardium.1 For image analysis and quantification of the LGE, several semi-automatic software packages already exist as medical products, however, at least a manual segmentation of endo- and epicardial contours by a professionally trained and experienced physician is typically still necessary.2 In recent years, a plethora of studies has proven the power of convolutional neural networks (CNNs) in pixel classification and semantic segmentation of myocardial tissue.3,4 The purpose of this study was to develop and train a convolutional neural network for semantic segmentation of myocardial scar tissue in 2D LGE images in short axis orientation.Methods
Image database & preparationA database was assembled containing 1702 images of contrast enhanced CMR imaging acquired on a 3T MR system (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). A 2D fast low-angle shot inversion recovery sequence in short axis orientation was applied 10 min after intraveneous injection of a Gd-based contrast agent. Myocardial contours and remote reference myocardium were segmented manually by an experienced operator using cvi42 (Circle Cardiovascular Imaging Inc, Calgary, CAN). Enhanced areas were obtained numerically by employing the n+5∙SD approach, as recommended by the Society for Cardiovascular Magnetic Resonance (SCMR).5 Additionally, areas of microvascular obstruction (MVO) were labeled manually as scar tissue. The final labels comprised a pixel classification for background, myocardium and scar. Both these labels and corresponding images were formatted uniformly to a size of 256 x 256. Finally, the dataset was divided in subsets for training (1200 images), validation (200 images) and testing (302 images).
Neural network
A CNN with a U-Net architecture and a ResNet34 backbone was trained using cross entropy as loss function. The 1cycle policy with cyclical momentum and learning rate was applied6 with an initial learning rate of 1e-3. Data augmentation of the training data was performed by random rotation, scaling and zooming. The model was trained for a total of 25 epochs with a continuous validation in order to evaluate the model during optimization.
Testing
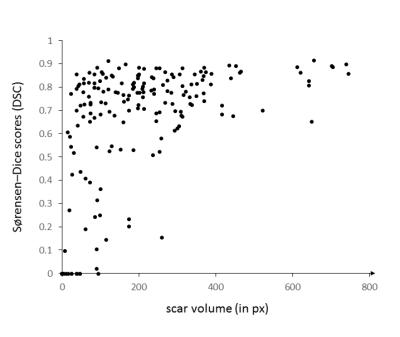
The performance of the proposed and trained artificial neural network was evaluated quantitatively by determining Sørensen–Dice scores (DSC) for scar tissue and for the myocardium. For scar tissue, image-specific DSC values were plotted against the total volume of the segments, respectively. For an overall estimation of the performance for each class, cardinalities were grouped across all instances before calculating an averaged DSC ($$$\overline{DSC}$$$Scar and $$$\overline{DSC}$$$Myo).
Results
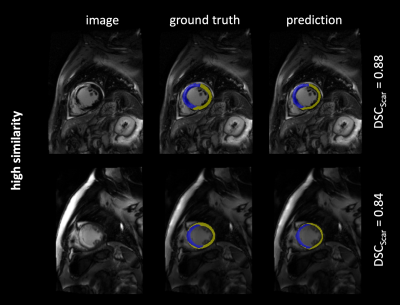
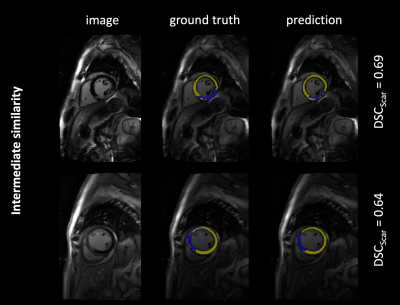
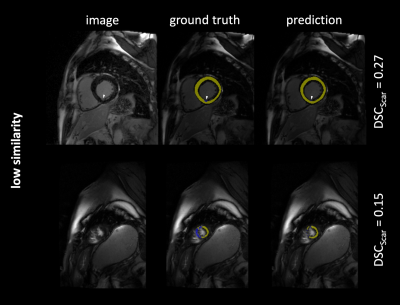
The 302 test images contained a total of 275 images with short axis images of the heart, and remaining 27 images of adjacent apical or basal slices. Scar tissue was present in 193 images (64 %). The model predictions yielded overall DSC as follows: $$$\overline{DSC}$$$Scar = 0.77, $$$\overline{DSC}$$$Myo = 0.81. Figure 1-3 exemplarily show images with superimposed labels for myocardium and scar tissue for high, medium and low similarity (respective DSC are indicated). The quantitative performance of the model showed a strong dependency towards the size of the scar area (Figure 2).Discussion
The proposed model was capable of automatically segmenting myocardium and scar tissue in LGE CMR images. Overall DSC was improved in comparison to previously proposed models,3 which might be predominantly substantiated by the larger training-dataset used in our study.A better performance in scar tissue was observed for larger volumes of scar tissue, with smaller hyper-enhanced areas yielding weaker DSC. The weakest performance was generally observed in apical and basal slices of the heart. This is not surprising, as differentiation of the endo- and epicardial borders is more complex in these areas, also for manual segmentation. Especially in these areas (but of course also globally) a larger image dataset would certainly improve the performance of the proposed neural network. Furthermore, a 3D network architecture, allowing for a volumetric interpretation may provide even higher precision.Conclusion
Deep learning based fully automatic segmentation of scar tissue in late gadolinium enhancement CMR is a promising approach to facilitate quantitative and fully automatic image analysis.Acknowledgements
- Grant support: German Ministry of Education and Research (BMBF, grant: 01EO1504).
- Circle Cardiovascular Imaging Inc for providing advanced access to export drawn labels.
References
1. Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N. Engl. J. Med. 2000.
2. Carminati MC, Boniotti C, Fusini L, et al. Comparison of image processing techniques for nonviable tissue quantification in late gadolinium enhancement cardiac magnetic resonance images. J. Thorac. Imaging. 2016.
3. Moccia S, Banali R, Martini C, et al. Development and testing of a deep learning-based strategy for scar segmentation on CMR-LGE images. Magn. Reson. Mater. Physics, Biol. Med. 2019.
4. Zhang N, Yang G, Gao Z, et al. Deep learning for diagnosis of chronic myocardial infarction on nonenhanced cardiac cine MRI. Radiology. 2019.
5. Schulz-Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J. Cardiovasc. Magn. Reson. 2013.
6. Smith LN. A disciplined approach to neural network hyper-parameters: Part 1 -- learning rate, batch size, momentum, and weight decay. 2018.
Figures