2226
Real-time Cine Cardiac Magnetic Resonance Imaging Using a Convolutional Neural Network1Department of Cardiology, Boston Children's Hospital and the Department of Pediatrics, Harvard Medical School, Boston, MA, United States, 2Department of Informatics, Technical University of Munich, Garching, Germany, 3Massachusetts Institute of Technology, Cambridge, MA, United States, 4Department of Pediatrics, Chiang Mai University, Chiang Mai, Thailand
Synopsis
We have developed and evaluated a new real-time acquisition and reconstruction technique for 2D steady-state free precession cine cardiac magnetic resonance imaging (MRI). Patient study is presented to validate the proposed technique.
Introduction
Accurate surveillance of ventricular function for deterioration and therapeutic response is an essential part of cardiac MRI.1 Ventricular function is typically assessed using a retrospectively electrocardiogram (ECG)-gated segmented k-space steady-state free precession (SSFP) cine acquisition.2 Respiratory motion artifact is minimized by having the patient repeatedly breath-hold as 1-3 slices of the image stack are acquired. This standard approach is relatively slow with a scan time of 3-5 minutes.3 Furthermore, variations in respiratory depth may lead to spatial misregistration between slices, introducing measurement errors in ventricular volumes.4 To address these shortcomings, we developed a highly under-sampled multislice real-time SSFP cine acquisition that was fast enough to cover the ventricles in a single breath-hold. The under-sampled data were then estimated by using a convolutional neural network-based (CNN) reconstruction algorithm. The real-time CNN images were compared with the conventional images in terms of sharpness and ventricular volume measurements.Methods
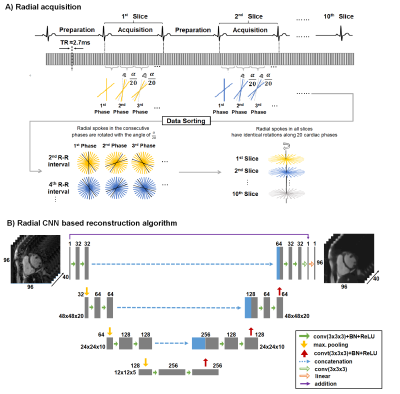
Real-time acquisition: A retrospectively ECG-gated SSFP sequence was developed to acquire 20 real-time images covering the entire cardiac cycle (Figure 1A). The first beat is used to saturate the signal from the myocardium within the imaging slice and the second beat is used to acquire the cine data for that slice. Each slice requires only one heartbeat for the acquisition of 20 consecutive real-time images. A radial k-space trajectory was employed to promote data sparsity and to cover more of k-space with fewer k-space lines. The radial k-space trajectory is rotated for each real-time image to take advantage of the evolution of data over time.5Subjects: Sixty-nine patients (32 females, median age 25 years) were prospectively recruited and consented for this study. On a 1.5T scanner (Philips, Achieva dStream), a ventricular short-axis stack was acquired with a fully-sampled segmented Cartesian acquisition and the proposed real-time radial 2D cine acquisition. Scan parameters for both acquisitions were FOV 256 x 256 mm, resolution 1.8 mm, slice thickness 7-8 mm, slice gap 0-2 mm, flip-angle 60°, TR/TE ≈2.7/1.4 ms, TFE-factor ≈14, 20 cardiac phases, and SENSE ×1. In the Cartesian acquisition, 10-13 breath-hold acquisitions each lasting ≈9 seconds were required to cover the ventricles. The real-time radial acquisition only required one breath-hold with a duration of ≈18 seconds to cover the ventricles.
Reconstruction: The raw data of segmented Cartesian and real-time radial acquisitions were retrospectively binned into 20 cardiac phases based on the ECG signal. View sharing was used only on the radial data to increase the number of spokes for each real-time image. The segmented Cartesian and real-time radial data were reconstructed by using FFT and iGRASP6 respectively. The reconstruction of all real-time radial slices using iGRASP took ≈11 hours per patient. To improve the image quality of iGRASP, a CNN-based reconstruction algorithm was developed. The CNN was based on a U-Net architecture (Figure 1B).7 We used 55 patients with paired data for training (input: radial magnitude images; ground truth: corresponding Cartesian magnitude images). All images were cropped to 96 × 96 pixels and normalized to a mean of zero and a standard deviation of one. The network was trained with batchsize of four using mean absolute error loss. L2-regularization was applied to prevent overfitting. The network was implemented in Keras using TensorFlow as the backend.8,9 The training was performed for 20 epochs with 10-fold cross-validation using the NADAM optimizer. The training time for the model was ≈10 hours on an NVIDIA Tesla M40 GPU. Six patient datasets were used for validation during the training and eight patient datasets were used for testing.
Analysis: The real-time radial test datasets reconstructed by the CNN were compared with the standard acquisition images in terms of spatial sharpness and ventricular volumes. Spatial sharpness is measured as the maximum gradient of normalized edge spread function of the signal intensity across the endocardial border of a midventricular slice.10 One clinician manually delineated the endocardial borders of the ventricles to calculate ventricular volumes.
Results
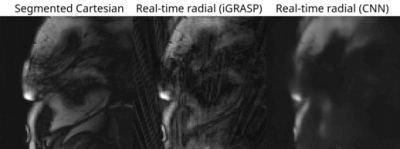
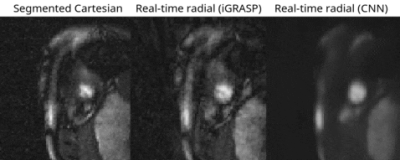
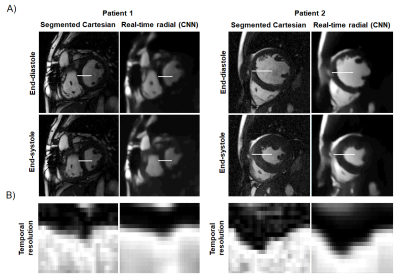
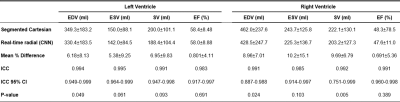
For the real-time radial images, the reconstruction of all slices using the CNN took ≈110 seconds per patient. CNN successfully removed all the streaking artifacts and reconstructed most of the cardiac anatomical features (Figures 2 and 3). Figure 4 compares the sharpness of segmented Cartesian and real-time radial images in two patients. Spatial sharpness was not significantly different between the segmented Cartesian and real-time radial images reconstructed by CNN (0.386±0.048 vs. 0.376±0.037, P-value =0.531). Table 1 compares the volumetric measurements between the segmented Cartesian and real-time radial images in 8 patients. There was an excellent agreement between the volumetric measurements of segmented Cartesian and real-time radial images (all intraclass-correlation-coefficients ≥0.983). The real-time radial volumetric measurements were all lower than the segmented Cartesian measurements (all mean-percent-differences ≤10.2%). The ejection fraction was comparable (P-value ≥0.389).Discussion & Conclusion
We developed a real-time ECG-gated radial 2D cine SSFP sequence fast enough for complete ventricular coverage in a single breath-hold and employed a CNN for image reconstruction. Compared to the standard segmented Cartesian acquisition, this technique yielded lower volumetric measurements, and comparable ejection fraction and spatial sharpness. Future work includes increasing the number of patients in the training dataset to improve the spatial and temporal sharpness of real-time radial images.Acknowledgements
The authors would like to thank all patients for their participation in the prospective cardiac MRI scans and our MRI technologists for performing all the scans. This work was supported by a fellowship within the PROMOS of the German Academic Exchange Service (DAAD) and American Heart Association (18CDA34110153).References
1. Semelka RC, Tomei E, Wagner S, Mayo J, Caputo G, O'Sullivan M, Parmley WW, Chatterjee K, Wolfe C, Higgins CB. Interstudy reproducibility of dimensional and functional measurements between cine magnetic resonance studies in the morphologically abnormal left ventricle. Am Heart J. 1990 Jun;119(6):1367-73. doi: 10.1016/s0002-8703(05)80187-5. PubMed PMID: 2141222.
2. Dehmer GJ, Lewis SE, Hillis LD, Twieg D, Falkoff M, Parkey RW, Willerson JT. Nongeometric determination of left ventricular volumes from equilibrium blood pool scans. Am J Cardiol. 1980 Feb;45(2):293-300. doi: 10.1016/0002-9149(80)90648-7. PubMed PMID: 7355738.
3. Moghari MH, Komarlu R, Annese D, Geva T, Powell AJ. Free-breathing steady-state free precession cine cardiac magnetic resonance with respiratory navigator gating. Magn Reson Med. 2015 Apr;73(4):1555-61. doi: 10.1002/mrm.25275. Epub 2014 Apr 28. PubMed PMID: 24777586.
4. Barkhausen J, Goyen M, Rühm SG, Eggebrecht H, Debatin JF, Ladd ME. Assessment of ventricular function with single breath-hold real-time steady-state free precession cine MR imaging. AJR Am J Roentgenol. 2002 Mar;178(3):731-5. doi: 10.2214/ajr.178.3.1780731. PubMed PMID: 11856708.
5. Chan YCI, Haji-Valizadeh H, Kim D, Brighenti M, Powell AJ, Moghari MH (2019) Retrospective ECG-gated radial 2D cine cardiac MRI, SCMR 22nd Annual Scientific Sessions, Bellevue, WA, USA, Feb 6-9, 2019
6. Feng L, Grimm R, Tobias Block K, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2013 Oct 18. doi: 10.1002/mrm.24980.
7. Ronneberger O, Fischer P, Brox T. U-Net: Convolutional networks for Biomedical Image Segmentation, 2015. Medical Image Computing and Computer-Assisted Intervention (MICCAI), Springer, LNCS, Vol.9351: 234--241, 2015, available at arXiv:1505.04597
8. Chollet F. et al. Keras. https://keras.io, 2015.et al. Keras. https://keras.io, 2015
9. Abadi M, Agarwal A, Barham P, Brevdo E, Chen Z, Citro C, Corrado GS, Davis A, Dean J, Devin M, Ghemawat S, Goodfellow I, Harp A, Irving G, Isard M, Jozefowicz R, Jia Y, Kaiser L, Kudlur M, Levenberg J, Mané D, Schuster M, Monga B, Sutskever I, Talwar K, Tucker P, Vanhoucke V, Warden P, Wattenberg M, Wicke, Yu Y, Zheng X. TensorFlow: Large-scale machine learning on heterogeneous systems, 2015. Software available from tensorflow.org.
10. Kording F, Schoennagel B, Lund G, Ueberle F, Jung C, Adam G, Yamamura J. Doppler ultrasound compared with electrocardiogram and pulse oximetry cardiac triggering: A pilot study. Magn Reson Med. 2015 Nov;74(5):1257-65. doi: 10.1002/mrm.25502. Epub 2014 Oct 30. PubMed PMID: 25359183.
Figures