2222
Longitudinal assessment of global longitudinal strain and hemodynamic forces in the diabetic mouse heart1Department of Biomedical Engineering, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands, 2Medis medical imaging systems B.V., Leiden, Netherlands, 3Faculty of Behavioural and Movement Sciences, Vrije Universiteit, Amsterdam, Netherlands
Synopsis
The current classification of heart failure based on diastolic and systolic dysfunction limits the understanding of the time course of heart failure with preserved left ventricular ejection fraction (HFpEF), and new parameters are warranted to assess cardiac function. Here, we assessed global longitudinal strain (GLS), as well as hemodynamic forces (HDF) in a mouse model of diabetes (db/db) at two time points, with the aim to study the prognostic value of these new parameters for cardiac dysfunction.
Introduction
The development of left ventricle (LV) remodeling associated with heart failure has so far been attributed to alteration in stresses on the myocardial fibers, leading to cell growth and proliferation. Aside from this biomechanical component, there are also intraventricular fluid dynamic changes during the process of cardiac remodeling that may even influence its progression (1). As LV remodeling alters functional parameters of the heart, it is important to identify suitable makers to detect remodeling in an early stage.Hemodynamic forces (HDF) caused by intraventricular pressure gradients (IVPG), are hypothesized to be an independent marker for cardiac function (2). While assessment of HDF values usually is complex and not widely available, a model has recently been introduced to estimate HDF using conventional 2-,3- and 4-chamber CINE-MRI (3). In this study, we used this model to assess changes in HDF and GLS, in addition to E’/A’-ratio (diastolic function) and EF(ejection fraction) as markers of cardiac function in a mouse model of diabetic cardiomyopathy at two time points. All animals were treated with either an antibiotic with established negative effect on cardiac function or a control(4).
Methods
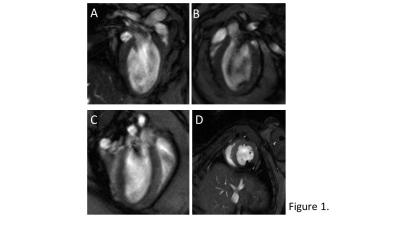
Seven (N=7) male diabetic (db/db) C57BL/Ks mice were measured twice: at 11 weeks and at 15-16 weeks of age. In an attempt to accelerate contractile dysfunction in the mice as shown in previous work (4), drinking water was supplemented with doxycycline (N=4), or amoxicillin as control antibiotic (N=3).Cardiac function was assessed under isoflurane anesthesia by cardiac MRI, with a 7.0 Tesla small animal scanner (MR Solutions, Guildford, UK) using a 38-mm-diameter mouse birdcage coil. Left ventricle (LV) systolic and diastolic function measurements were performed using short-axis multi-slice CINE-MRI and a single-slice midventricular acquisition, respectively. (4). GLS and HDF measurements were assessed using 2-, 3-, and 4-chamber long-axis views (Figure 1). Both diastolic function, as well as GLS and HDF measurements used single-slice high frame rate retrospectively gated compressed sensing (CS) accelerated CINE MRI acquisitions, as previously described (5) with the following sequence parameters: TR/TE = 7/2.35 ms, flip angle = 15°, FOV = 30×30 mm2, matrix size = 192×192, slice thickness = 1 mm, number of k-space repetitions = 400, total acquisition time = 13 min.
Off-line reconstruction of all retrospectively gated CINE acquisitions was performed in MATLAB 8.1 (The Mathworks, Natick, MA, USA) using custom-built routines. In short, imaging data was binned into 32 frames per cardiac cycle and reconstructed by a compressed sensing algorithm using the Berkeley Advanced Reconstruction Toolbox (BART). Cine movies were subsequently analyzed using the Qmass and Qstrain plugins in the MEDIS suite MR software (Leiden, The Netherlands). Besides endocardial contours, mitral and aortic valve diameters were assessed as input for HDF calculations.
The ratio between the dimensionless root mean square of HDF in the lateral-septal and apical-basal direction, was measured as force ratio [%] for systolic as well as diastolic phase of the cardiac cycle to determine a shift in force alignment from the longitudinal to transverse direction (Figure 2). GLS was measured over time as the average GLS of the whole heart and peak values were used for statistical analysis. Values are mean±std.
End-diastolic and end-systolic volumes of the LV were used to calculate EF as a measure for systolic function. Diastolic function was measured as the ratio of the early or elastic (E’) and atrial (A’) filling rates.
Results
A typical example of the self-gated retrospectively triggered mouse CINE MRI is given in Figure 1. Cardiac MRI confirmed that the untreated db/db animals suffered from heart failure with preserved ejection fraction (HFpEF), as the E’/A’ ratio was lower and EF similar compared to previously reported control data(4).In this study, prior to treatment, the animals (N=7) had an EF of 72.55±7.14%, and an E’/A’ ratio of 2.01±0.36. The doxycycline and amoxicillin treated animals had an EF of 74.72±9.01% and 71.74±5.16% and an E’/A’ ratio of 2.09±0.51 and 1.69±0.27 respectively.
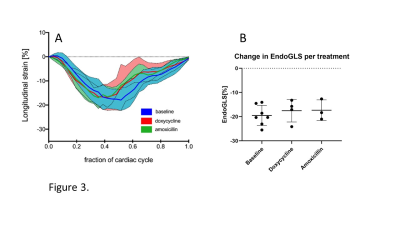
Figure 3 shows GLS strain throughout the cardiac cycle, with the peak occurring during end-systolic phase. Peak GLS values of the doxycycline(-17.53±4.68%) and amoxicillin(-17.34±4.27%) treated animals became slightly less negative compared to baseline measurement (-19.48±4.19%).
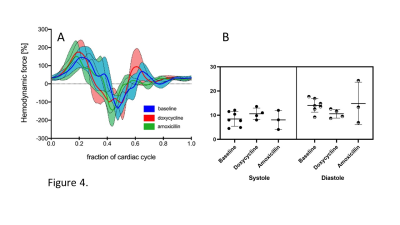
The hemodynamic force ratio percentage for the systolic and diastolic phase phase at baseline was 8.44±3.03 and 14.07±2.84% respectively. Furthermore, mean systolic and diastolic force ratio’s for amoxicillin(8.07±3.95 and 14.83±8.80%) treated animals, were comparable to the baseline. The systolic force ratio for the doxycycline (10.6±2.35%) treated group was increased compared to baseline measurements. However the diastolic force ratio for this group(10.6±1.81%) is decreased compared to the baseline(Figure 4).
Discussion & Conclusion
We showed the feasibility of measuring GLS and HDF parameters in a mouse model of cardiac dysfunction using high frame rate retrospectively gated 2-,3-, and 4-chamber CINE MRI. While our current model did not show large changes in GLS and HFD parameters over time, we believe these techniques may add positive value to cardiac function assessment in mouse models of cardiac dysfunction. As a decrease was measured between treatment and baseline for GLS and increase in systolic force ratio was measured for the doxycycline group, which could indicate a subendocardial fiber dysfunction and a shift in force alignment to the lateral septal direction(6).Acknowledgements
No acknowledgement found.References
(1) Domenichini, Federico, and Gianni Pedrizzetti. "Hemodynamic forces in a model left ventricle." Physical Review Fluids 1, no. 8 (2016): 083201.
(2) Arvidsson, Per M., Johannes Töger, Gianni Pedrizzetti, Einar Heiberg, Rasmus Borgquist, Marcus Carlsson, and Håkan Arheden. "Hemodynamic forces using four-dimensional flow MRI: an independent biomarker of cardiac function in heart failure with left ventricular dyssynchrony?." American Journal of Physiology-Heart and Circulatory Physiology 315, no. 6 (2018): H1627-H1639.
(3) Pedrizzetti, Gianni, Per M. Arvidsson, Johannes Töger, Rasmus Borgquist, Federico Domenichini, Håkan Arheden, and Einar Heiberg. "On estimating intraventricular hemodynamic forces from endocardial dynamics: a comparative study with 4D flow MRI." Journal of biomechanics 60 (2017): 203-210.
(4) Wüst, Coolen, Alizadeh Tazehkandi, Daal, Houtkooper and Strijkers (2019). The antibiotic doxycycline compromises cardiac mitochondrial and contractile function in diabetic mice. In Proceedings of the Annual Meeting ISMRM 2018, p. 1088.
(5) Motaal, Abdallah G., Bram F. Coolen, Desiree Abdurrachim, Rui M. Castro, Jeanine J. Prompers, Luc MJ Florack, Klaas Nicolay, and Gustav J. Strijkers. "Accelerated high‐frame‐rate mouse heart cine‐MRI using compressed sensing reconstruction." NMR in Biomedicine 26, no. 4 (2013): 451-457
(6) Claus, Piet, Alaa Mabrouk Salem Omar, Gianni Pedrizzetti, Partho P. Sengupta, and Eike Nagel. "Tissue tracking technology for assessing cardiac mechanics: principles, normal values, and clinical applications." JACC: Cardiovascular Imaging 8, no. 12 (2015): 1444-1460.
Figures