2215
Sodium MRI of the Rat Heart at 9.4 T using a Quadrature Birdcage
Laura Boehmert1, Helmar Waiczies2, Andre Kuehne2, Celal Oezerdem1, Sonia Waiczies1, Ludger Starke1, Min-Chi Ku1, Andreas Pohlmann1, Paula Ramos Delgado1, Erdmann Seeliger3, and Thoralf Niendorf1,2,4,5
1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 2MRI.TOOLS GmbH, Berlin, Germany, 3Institute of Vegetative Physiology, Charité University Medicine, Berlin, Germany, 4DZHK (German Centre for Cardiovascular Research), partner site Berlin, Berlin, Germany, 5Experimental and Clinical Research Center, a joint cooperation between the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany
1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 2MRI.TOOLS GmbH, Berlin, Germany, 3Institute of Vegetative Physiology, Charité University Medicine, Berlin, Germany, 4DZHK (German Centre for Cardiovascular Research), partner site Berlin, Berlin, Germany, 5Experimental and Clinical Research Center, a joint cooperation between the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine, Berlin, Germany
Synopsis
We propose probing of tissue sodium concentrations in the heart using 23Na MRI for adding a very useful dimension to our understanding of cardiac disorders. Changes in the sodium concentration might be indicative of early pathophysiological changes in such diseases. This work focuses on the development, evaluation and application of a quadrature birdcage RF resonator tailored for cardiac 23Na MRI at 9.4T with the goal to provide a uniform excitation profile.
Introduction
The broad role of sodium in cardiac physiology suggests a wide range of questions for further in vivo cardiac investigations. Availability of sodium MRI technology could provide mechanistic insights and add a useful dimension to our understanding of cardiac diseases. Preclinical cardiac 23Na MRI has been performed in isolated and perfused rat hearts1-3, in mice using vertical magnets at 17.6T4 and in vivo in rats using 7T5. Transmission (B1+) field homogeneity and a uniform sensitivity profile are beneficial for sodium quantification. Recognizing this opportunity this work focuses on the development, evaluation and application of a quadrature birdcage RF resonator tailored for cardiac 23Na MRI at 9.4T, with the goal of providing a uniform excitation profile with less than 2% standard deviation in B1+ over a central axial cross-section with a diameter of 40mm. The suitability of the RF resonator for 23Na MRI of the heart is demonstrated in rats, as a mandatory precursor to broader studies in experimental models of cardiac diseases.Methods
A 16 rungs low-pass quadrature birdcage coil (l=100mm, d=62mm) was designed. The birdcage was manufactured from a 35 µm-copper coated substrate (FR-4) with a thickness of 0.1mm. Each port was equipped with a tuning and matching circuit and was additionally decoupled. MR experiments were conducted on a 9.4T animal scanner (Biospec, Bruker, Germany). Phantom 23Na images were acquired with a FLASH-sequence (quantification phantom: TR/TE=50ms/2ms, matrix-size=128x128, FOV=(64x64)mm2, slice thickness=10mm, FA=70°, TAcq=30min; cylindrical tissue-mimicking phantom: 50ms/2ms, matrix-size=64x64, FOV=(64x64)mm2, slice thickness=5mm, FA=70°, TAcq=30min). Animal experiments were approved by the Animal Welfare Department of Berlin’s State Office of Health and Social Affairs and were performed in accordance with the German Animal Protection Law and the guidelines to minimize discomfort to animals (86/609/EEC). The rat was placed on a water-circulating heated holder to ensure constant body temperature (37°C) and was anesthetized with 1.2g/kg body weight urethane (Sigma Aldrich/Merck). Body temperature and breathing rate were constantly monitored (Model 1025, SA Instruments, NY). For in vivo MRI, a 1H resonator of similar geometry was used to obtain anatomical reference images and for magnetic field shimming. The 1H and 23Na resonator were inserted from the back of the scanner and placed in the isocenter without moving the animal. Anatomical 1H scans were performed using a FLASH-sequence (TR/TE=31.5ms/1.7ms, matrix-size=192x192, FOV=(46x46)mm2, FA=80°, slice thickness=1mm, TAcq=5min). 23Na in vivo imaging of the rat heart was conducted with a FLASH-sequence (TR/TE=50ms/1.6ms, matrix-size=46x46, FOV=(46x46)mm2, FA=80°, slice thickness=5-10mm, TAcq=30-120min).Results
The loaded and unloaded quality-factor was estimated for the tissue mimicking phantom (QL=173; QU=254) resulting in a Q-ratio (QU/QL) of 1.47, which indicates dominance of the probe noise. The reflection coefficients of both channels (S11;S22) were <‑45dB. Evaluation of the birdcage was performed using the quantification phantom retrofitted with NMR tubes filled with NaCl (cNaCl=100-400mM) for calibration (Figure 1a). The corresponding 23Na MR image is shown in Figure 1b. Figure 1c demonstrates the linear dependence of the SNR versus the sodium concentration. A profile plot along the line for an axial (Figure 1d), coronal (Figure 1e) and sagittal 23Na image is shown in Figure 1f. The RF resonator provides a transmission field homogeneity with a standard deviation of less than 2% over a central axial cross section with a diameter of 40mm and a penetration depth that affords in vivo 23Na MR imaging of the rat heart at 9.4T (Figure 2). Figure 3a,d show the 1H reference image of the short axis view of the rat heart. Figure 3b,e illustrate the corresponding 23Na image for a spatial resolution of (1x1x5)mm³ and (1x1x10)mm³. Figure 3c and f-i demonstrate the 23Na/1H overlay for the two spatial resolutions and for acquisition times of 30 to 120min. A higher concentration of sodium is seen in the blood pool within the left and right ventricles, when compared to the myocardium. A clear delineation of 23Na concentrations is observed at the septal wall. A 23Na SNR of 9-14 was obtained for the myocardium. 23Na SNR was 11-18 and 12-19 for the left and right ventricle respectively. The blood-myocardium CNR was found to be 3-5.Discussion
This work shows a uniform excitation and receive intensity profile of a quadrature 23Na birdcage resonator and demonstrates its suitability for in vivo cardiac imaging in small rodents at 9.4T. Our SNR results obtained for sodium imaging of the rat heart (SNRLV=11, SNRRV=12, SNRmyocardium=9) using a spatial resolution of (1x1x5)mm3 and a scan time of 30min compare well to those reported for sodium MRI of the mouse heart at 17.6T4 when only the differences in magnetic field strength6, spatial resolution, and acquisition time are taken into account: SNRLV=16, SNRRV=14.5, SNRseptum=9.8.Conclusion
This work presents a 23Na quadrature birdcage volume resonator with a uniform transmission field and demonstrates for the first time the feasibility of sodium imaging of the rat heart in vivo at 9.4T. Using 23Na MRI in translational research for probing sodium concentration in the heart in vivo stands to make a critical contribution to deciphering the complex mechanisms underlying cardiac diseases. Our work provides the technological bases for detecting changes in tissue sodium concentration indicative of early pathophysiological processes, with 23Na MRI playing a decisive role in the understanding and monitoring of cardiac diseases.Acknowledgements
No acknowledgement found.References
- Jansen MA, Van Emous JG, Nederhoff MG, Van Echteld CJ. Assessment of myocardial viability by intracellular 23Na magnetic resonance imaging. Circulation. Nov 30 2004;110(22):3457-3464.
- Weidensteiner C, Horn M, Fekete E, Neubauer S, von Kienlin M. Imaging of intracellular sodium with shift reagent aided (23)Na CSI in isolated rat hearts. Magnetic resonance in medicine. Jul 2002;48(1):89-96.
- Aguor EN, van de Kolk CW, Arslan F, et al. 23Na chemical shift imaging and Gd enhancement of myocardial edema. The international journal of cardiovascular imaging. Feb 2013;29(2):343-354.
- Neuberger T, Greiser A, Nahrendorf M, Jakob PM, Faber C, Webb AG. 23Na microscopy of the mouse heart in vivo using density-weighted chemical shift imaging. MAGMA. Dec 2004;17(3-6):196-200.
- Jansen MA, Kägebein U, Wetterling F, Gray GA, I. M. In vivo Cardiac cine 23Na MRI in rats. Intl. Soc. Mag. Reson. Med. Salt lake City, USA 2013.
- Pohmann R, Speck O, Scheffler K. Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 tesla using current receive coil arrays. Magnetic resonance in medicine. Feb 2016;75(2):801-809.
- Cunningham CH, Pauly JM, Nayak KS. Saturated double-angle method for rapid B1+ mapping. Magnetic resonance in medicine. Jun 2006;55(6):1326-1333.
Figures
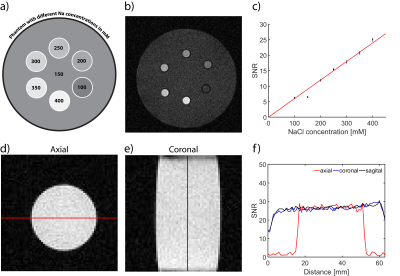
Figure 1: Evaluation and
quantification of the RF coil performance. (a) Experimental setup of the
validation phantom containing NMR tubes with sodium concentrations ranging from
100mM to 400mM. (b) Cross-section of the corresponding 23Na image.
(c) Calibration of SNR versus NaCl concentration in the NMR tubes and phantom
(r2=0.99, slope=0.06(mM)-1). (d-e) axial and coronal 23Na
MR views of the
cylindrical tissue mimicking phantom.
(f) SNR profiles along the lines highlighted in d and e demonstrating the
signal uniformity facilitated by the RF volume resonator.
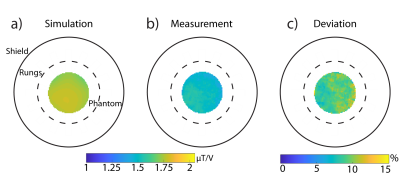
Figure 2: Comparison of the
simulated (a) and measured (b) transmission (B1+)
field (in mT/V) obtained for an axial cross-section of the tissue
mimicking phantom. The experimental B1+ field was
acquired with the double angle method7. (c) Difference B1+
map (Deviation = (Simulation-Measurements)/Simulation) between the measurement
and the simulation.
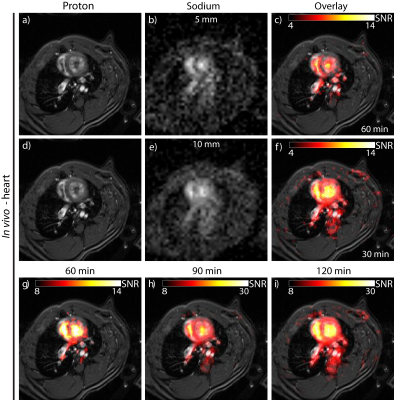
Figure 3: In vivo 23Na
image of a rat heart obtained by using a FLASH technique. (a, d) show the
anatomical 1H heart reference image. (b, e) the corresponding
interpolated 23Na image and (c) the overlay of the 1H/23Na
images. The cardiac image in (f-i) was acquired in 30 - 120min with a
resolution of (1x1)mm2 and 10mm slice thickness and (c) in 60min
using a slice thickness of 5mm. 23Na acquisitions were performed without using
cardiac or respiratory gating.