2194
High Resolution Simulation and Measurement of Phase-Specific B0 Field Conditions Across the Human Cardiac Cycle1Department of Biomedical Engineering, Columbia University, New York, NY, United States, 2Chair of Cellular and Molecular Imaging, Comprehensive Heart Failure Center, University Hospital Wuerzburg, Wuerzburg, Germany, 3Department of Cardiovascular Imaging, Comprehensive Heart Failure Center, University Hospital Wuerzburg, Wuerzburg, Germany, 4Department of Radiology, Columbia University, New York, NY, United States
Synopsis
B0 inhomogeneity leads to dark band artifacts in cardiac MRI, in particular with the use of SSFP pulse sequences. Limited spatial resolution of MRI-derived B0 maps prevents the systematic analysis of the problem and the development of optimized B0 shim strategies. Here we demonstrate the potential for simulating both overall and cardiac phase-specific B0 field conditions in the human heart at 3 T at high spatial resolution from anatomical MRI. The results are validated by high-resolution B0 field mapping in the same subjects. This approach is expected to develop population-specific B0 shim strategies from readily available anatomical MRI libraries.
Introduction
Heart disease is the leading cause of death among all ages in the United States1. Cardiovascular Magnetic Resonance imaging (CMR) with the use of steady-state free precession (SSFP) pulse sequences is a widely-used method to diagnose heart diseases and assess cardiac function2,3. SSFP-based CMR provides excellent image contrast between the myocardium and the blood pool3, making it a suitable clinical tool. Their sensitivity to B0 inhomogeneities can lead to signal loss and so-called dark band artifacts4,5. B0 field inhomogeneity in the human heart arises primarily from magnetic susceptibility differences between the lungs and heart tissue6 and is furthermore modulated over the cardiac cycle7,8 as geometric conditions change.We have shown previously that overall B0 conditions in the human heart can be predicted from anatomical CT images9. In this work, we demonstrate the potential for simulating cardiac phase-specific B0 field conditions in the human heart at 3 T at high spatial resolution from anatomical MRI of the subject at hand. The results are validated by high-resolution B0 field mapping in the same subjects.
Methods
Cardiac phase-specific B0 conditions were simulated based on tissue masks created from the magnitude images of the cardiac phase-specific B0 maps averaged across the two echo times (for increased SNR) assuming -9 ppm and 0 ppm susceptibility for tissue and air, respectively, as described previously9. To minimize finite volume effects in the simulations, the cardiac phase-specific tissue masks were extended to the whole thoracic area using the larger FOV GRE data which was acquired by a non-triggered anatomical GRE scan with 3 mm isotropic resolution and FOV = 450 x 382 x 312 mm3 in free breathing. The B0 conditions across the segmented heart ROI were decomposed in zero through third order spherical harmonics (SH) with B0DETOX10.For experimental validation, cardiac phase-specific B0 field maps were acquired from 5 healthy male subjects (age: 31 ± 4) on a 3 T Prisma MR scanner (Siemens, Erlangen, Germany) using an ECG triggered gradient echo (GRE) sequence in 3 breath holds with TR/TE1/TE2 = 79/2.04/4.34 ms, isotropic spatial resolution of 3 mm and FOV = 195 x 195 x 108 mm³. Images were acquired with a varying number of phases per cardiac cycle (min/max/mean = 6/11/8.8) depending on the subjects’ heart rate (mean ± std: 66 ± 15 bpm). For every subject and cardiac phase, the region of interest (ROI) of the heart was segmented individually using in-house software (written in MATLAB, MathWorks, Natick, MA, USA) and then processed using the Medical Imaging Interaction Toolkit software11.
Results
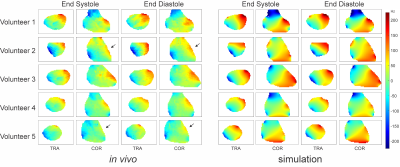
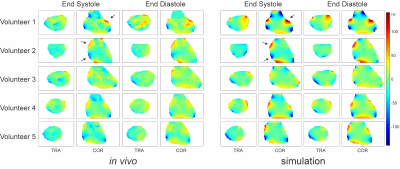
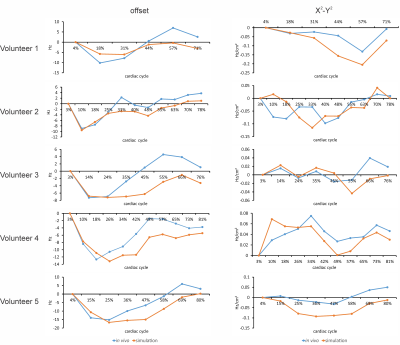
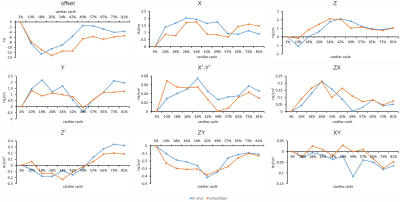
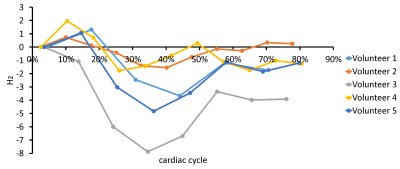
Simulated high-resolution cardiac phase-specific B0 field distributions based on regional anatomical features (Figure 1) and the corresponding B0 maps measured directly in the same subjects (Figure 2) exhibited spatial patterns typical for the human heart including localized high-amplitude distortions. Simulated and measured high-level B0 patterns were consistent both spatially, e.g. when decomposed in higher order SH shapes, as well as temporally across the cardiac cycle in all subjects. Note the combination of common temporal behavior across all subjects and distinct subject-specific features. Low-level B0 features somewhat differed, for instance, the linear Z-gradient (3.2%) which is hypothesized to be due to the applied first-order default shim applied in the experiments or larger scale magnetic susceptibility conditions, i.e. overall body anatomy beyond the chest area, not considered in the B0 field simulations. This relative temporal progression is plotted for all subjects for selected exemplary SH coefficients in Figure 3. In vivo measurements largely resemble the theoretically predicted B0 field behavior for all SH coefficients up to the second SH order, as shown in Figure 4. Different dynamic changes of B0 field inhomogeneities were observed for all subjects during the cardiac cycle (Figure 5).Discussion
Here we present a detailed spatio-temporal analysis of the B0 field conditions in the human heart at 3 T. Theoretical simulations employing regional anatomical features enabled the high-resolution and noise-free characterization of the B0 field conditions and were validated by direct experimental B0 mapping in the same subjects. A consistent subject-independent oscillatory progression of the spatial B0 distribution observed throughout the cardiac cycle is expected to allow the development of generalized B0 shim approaches valid across populations, whereas the theoretical prediction of detailed anatomy-based B0 variations will be used to derive advanced subject-specific B0 shim strategies. Potential applications include the development of optimal static B0 shimming (minimizing B0-induced CMR artifacts across the cardiac cycle) as well as cardiac phase-specific real-time B0 shimming, potentially without the need to acquire cardiac phase-specific B0 maps for the subject at hand.Acknowledgements
Financial support was obtained from the German Ministry of Education and Research (BMBF) under grants: 01EO1004 & 01EO1504.
Cardiac B0 field mapping was done at Zuckerman Mind Brain Behavior Institute MRI Platform at Columbia University, a shared resource.
References
1. Benjamin EJ et al., Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56-e528.
2. Earls JP, Ho VB, Foo TK, Castillo E, Flamm SD. Cardiac MRI: Recent Progress and Continues Challenges. J Magn Reson Imaging. 2002;16(2):111-127.
3. Wieben O, Francois C, Reeder SB. Cardiac MRI of ischemic heart disease at 3 T: Potential and challenges. Eur J Radiol. 2008;65(1):15-28.
4. Ferreira PF, Gatehouse PD, Mohiaddin RH, Firmin DN. Cardiovascular magnetic resonance artefacts. J Cardiovasc Magn Reson. 2013;15(1):41.
5. Schär M, Kozerke S, Fischer SE, Boesiger P. Cardiac SSFP Imaging at 3 Tesla. Magn Reson Med. 2004;51(4):799-806.
6. Atalay MK, Poncelet BP, Kantor HL, Brady TJ, Weisskoff RM. Cardiac Susceptibility Artifacts Arising From the Heart-Lung Interface, Magn Reson Med. 2001;45(2):341-345.
7. Kubach MR, Bornstedt A, Hombach V, Merkle N, Schär M, Spiess J, Nienhaus GU, Rasche V. Cardiac phase-specific shimming (CPSS) for SSFP MR cine imaging at 3 T, Phys Med Biol. 2009;54(20):N467-78.
8. Mattar W, Juchem C, Terekhov M, Schreiber L. Multi-Coil B0 shimming of the Human Heart: A Theoretical Assessment, Proc. ISMRM (2016), p. 1151.
9. Shang Y, Theilenberg S, Mattar W, Terekhov M, Jambawalikar SR, Schreiber L, Juchem C. High Resolution Simulation of B0 Field Conditions in the Human Heart Based on Segmented CT Images. Proc Int Soc Magn Reson Med 2019, 2184.
10. Juchem C. B0DETOX - B0 Detoxification Software for Magnetic Field Shimming. Columbia TechVenture (CTV), License CU17326. http://innovation.columbia.edu/technologies/cu17326_b0detox (2017).
11. Wolf I, Vetter M, Wegner I, Böttger T, Nolden M, Schöbinger M, Hastenteufel M, Kunert T, Meinzer HP. The medical imaging interaction toolkit. Med Image Anal. 2005;9(6):594-604.
Figures