2193
Fast 3D Whole Heart Imaging using Seiffert Spirals1Core Facility Small Animal Imaging (CF-SANI), Ulm University, Ulm, Germany, 2Department of Internal Medicine II, University Ulm Medical Center, Ulm, Germany
Synopsis
The overall duration of acquiring a Nyquist sampled 3D dataset can be significantly shortened by enhancing the efficiency of k-space sampling. This can be achieved by increasing the coverage of k-space for every trajectory interleave. The presented and adapted acquisition scheme using Seiffert Spirals can be a valuable tool for applications in which scan durations are strictly limited e.g. in the case of 3D ECG gated cardiovascular imaging. In this context we apply the Seiffert Spirals to isotropic whole heart imaging with overall scan times of 350 heart beats for a resolution of (1.25 mm)3.
Introduction
Cardiac and vascular anatomies of patients with congenital heart disease are often complex and require imaging in many non-standard anatomical planes. Therefore, three-dimensional navigator based and ECG triggered sequences are often used to acquire images of the entire heart with the possibility of retrospectively reconstructing arbitrary anatomical planes. Unfortunately, these suffer from intrinsically long acquisition times to achieve sufficient and diagnosable in-plane resolutions.This work applies the imaging concept of Seiffert Spirals to cardiovascular imaging in order to achieve a fast and low-discrepancy coverage of $$$k$$$-space with the possibility of further accelerating the sequence in combination with non-linear optimisation techniques such as Compressed Sensing.
Methods
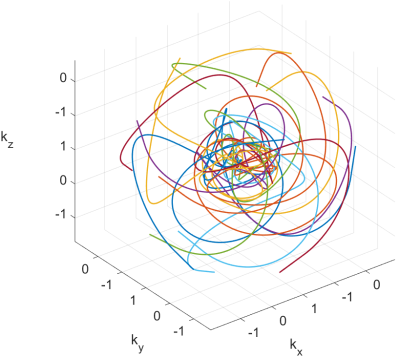
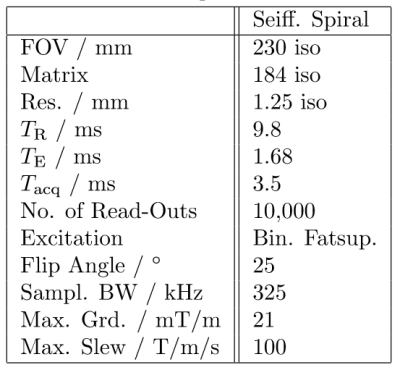
The Seiffert spirals are iteratively constructed according to 1 and adapted to a gradient system with a maximum amplitude of 21 mT/m and a read-out duration of 3.5 ms per interleave to reduce off-resonance effects. Additionally, the radial increment was set to 1.3, based on the energy distribution of previously acquired cartesian whole heart images. In total, the sequence consists of 10,000 interleaves to sample an isotropic volume of 230 mm$$$^3$$$ with a resolution of 1.25$$$^3$$$ mm$$$^3$$$. Further scan parameters are given in table 1. Figure 1 shows exemplarily the $$$k$$$-space coverage of 15 interleaves with the increased sampling density around the centre of $k$-space due to the radial increment.In order to minimise motion and off-resonance artefacts, the sequence was combined with ECG gating and a navigator based compensation of respiratory motion as well as with spectral selective fat suppression pulses. Enhanced $$$T_2$$$ contrast was generated by the application of $$$T_2$$$-preparation pulses once per heart cycle. Ten interleaves of the Seiffert trajectory were acquired during each myocardial resting phase, leading to a total requirement of 1,000 heart beats.
All images were acquired on a 1.5\,T whole body MRI system (Achieva 1.5T, Philips Healthcare, Best, The Netherlands) using a 5-channel cardiac coil. Further acceleration was achieved by 2.85-fold (polar) undersampling in combination with a Compressed Sensing reconstruction, leading to only 350 required heart beats.
Results
Figure 2 shows images of all three anatomical planes, once reconstructed using the entire dataset of 1,000 heart beats and 10,000 interleaves (left-hand side) and once with 2.85-fold undersampling in combination with Compressed Sensing (right-hand side). Echocardiographical planes can easily be reconstructed from the whole heart dataset shown in figure 2. Figure 3 shows a corresponding four-chamber and two-chamber view as well as a short axis slice.Discussion and Conclusion
The acquired whole-heart images, fully sampled and with 2.85-fold undersampling show clear delineation of the myocardium. Isotropic resolutions further enable arbitrary reconstructions of anatomical planes. A further decrease in scan duration can be achieved by restricting the sequence to anisotropic field of views by adapting the sampling bandwidth of each interleave with respect to the position of the corresponding Fibonacci point1.Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 667192.
The authors thank the Ulm University Centre for Translational Imaging MoMAN for its support.
References
[1] Speidel T., Metze P. and Rasche V. Efficient 3D Low-Discrepancy k-Space Sampling Using Highly Adaptable Seiffert Spirals. IEEE Trans Med Imaging. 38, 1833-1840, 2019.Figures