2186
Magnetic resonance imaging for assessment of aortic coarctation impact on cardiac function
El-Sayed H Ibrahim1, Pierre Croisille2, and John LaDisa3
1Medical College of Wisconsin, Milwaukee, WI, United States, 2Jean-Monnet University, Lyon, France, 3Marquette University, Milwaukee, WI, United States
1Medical College of Wisconsin, Milwaukee, WI, United States, 2Jean-Monnet University, Lyon, France, 3Marquette University, Milwaukee, WI, United States
Synopsis
Coarctation of the aorta (CoA) is a constriction of the descending thoracic aorta and is one of the most common congenital cardiovascular defects affecting 5,000-8,000 births annually in USA. Patients with CoA can have hyperdynamic and remodeled left ventricle from increased afterload. The mechanisms of morbidity from CoA are difficult to study in clinical setting due to the patients’ heterogeneity from confounding variables and concomitant anomalies. To remove these barriers, we adapted a novel rabbit model of CoA, which we scanned using MRI to study CoA-induced alterations in global and regional cardiac function and compare results to measurements from controls.
Introduction
Coarctation of the aorta (CoA) is a constriction of the descending thoracic aorta and is one of the most common congenital cardiovascular defects affecting 5,000-8,000 births annually in USA. Patients with CoA can have a hyperdynamic and remodeled left ventricle (LV) from increased afterload. The mechanisms mediating persistent morbidity from CoA are difficult to study in a clinical setting due to relatively small number of patients at each center annually, their heterogeneity from confounding variables, and concomitant anomalies. To remove these barriers, we adapted a novel rabbit model of CoA to control for the variability seen in humans (CoA severity, duration and subject age) without concomitant anomalies such as bicuspid aortic valves and septal defects. In this study, we use this rabbit model to study CoA-induced alterations in cardiac function (both global and regional) and compare results to measurements from controls.Methods
New Zealand white rabbits ~10-week old and weighing ~1.0 kg underwent CoA of the proximal descending thoracic aorta. Briefly, a 1.6-mm diameter stainless steel wire was used with silk suture to mimic untreated CoA. This diameter wire resulted in a blood pressure gradient (BPG) near the putative value for intervention in patients diagnosed with CoA at the experimental end point of 32 weeks. Anesthetized rabbits (CoA = 7; control = 4) were scanned at 32 weeks in the supine, head first position on a GE 3T MRI Premier scanner (GE Healthcare, Waukesha, WI) using an 18-channel knee coil and peripheral gating. Monitoring equipment used to ensure an adequate level of anesthesia included an external pulse oximeter and core temperature sensor approved for use in the MR environment. Cardiac triggering was obtained using a peripheral pulse oximeter attached to the right ear that also provided heart rate. Both cine and tagging images were acquired during free-breathing to obtain short-axis (SAX) and long-axis (LAX) slices covering the heart. As a relatively small-size animal is imaged on a human MRI scanner, imaging parameters were optimized to improve spatial and temporal resolutions and signal-to-noise ratio (SNR), while minimize motion and off-resonance artifacts. Representative cine and tagging images are shown in Figure 1. Optimal imaging parameters for the cine sequence were: TR=4.5ms, TE=1.6ms, flip angle=20°, FOV =160x160mm2, matrix=256x256, slice thickness=3mm, #averages=3, #heart phases=20 (~16ms temporal resolution), readout-bandwidth=488Hz/pixel. Optimal imaging parameters for the tagging sequence were similar to those in cine imaging, except for: TR=7.6ms, TE=4.4ms, flip angle=6°, #averages=4, readout-bandwidth=390Hz/pixel, tag spacing=3mm. The cine images were analyzed to measure ejection fraction (EF), end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV), cardiac output (CO), and mass. The tagged images were analyzed using Sinusoidal Modeling (SinMod) technique to measure circumferential (Ecc) and longitudinal (Ell) myocardial strains.Results
Animal weight / heart rate were 2.9±0.1 kg / 169±32 bpm and 3.1±0.1 kg / 183±17 bpm in the CoA and control rabbits, respectively. The results showed slightly increased EF in CoA rabbits compared to controls (56.3±6 % vs. 53.3±7.2 %, respectively). While ESV was similar in both groups (1.8±0.4 ml and 1.7±0.4 ml), EDV (4.2±0.7 ml vs. 3.6±0.4 ml), SV (2.3±0.4 ml vs. 1.9±0.2 ml), and CO (0.39±0.07 l/m vs. 0.35±0.02 l/m) were slightly increased in the CoA rabbits compared to controls. Myocardial mass was significantly increased in the CoA rabbits compared to controls (5±1.6 g vs. 3.4±0.4 g; P < 0.05). Strain results showed poorer myocardium contractility in CoA compared to control rabbits (Figures 2 and 3). Ecc (global / basal / mid-ventricular / apical) measurements were -19±2.7% / -21±4.7% / -17±3.9% / -18±3.2% and -21±7.7% / -23±8.5% / -19±5% / -28±9% in the CoA and control rabbits, respectively. Ell (global / basal / mid-ventricular / apical) measurements were -20±1.7% / -16±6.1% / -20±4% / -25±2.9% and -23±1.7% / -13±0.8% / -25±3.9% / -31±7.4% in the CoA and control rabbits, respectively. Statistical significance between strain measurements in CoA and control rabbits could not be established due to small number of studied animals.Discussion
Although remodeling of the LV in response to pressure overload has been studied in numerous animal models, there is a paucity of data using a clinically representative BPG that resembles CoA in patients to investigate the impact on cardiac function and myocardial contractility. This study investigates the impact of CoA on cardiac function using a rabbit model of CoA that controls for the variability seen in humans without concomitant anomalies. CoA rabbits showed normal global function, which could be understood in light of ventricular remodeling and hypertrophy to maintain cardiac output. Nevertheless, strain analysis showed poorer regional cardiac function and reduced myocardial contractility compared to the control rabbits. Therefore, regional cardiac function could allow for early detection of subclinical cardiac dysfunction in CoA despite normal EF.Conclusion
Cardiac MRI strain analysis is a valuable tool for evaluating the effect of CoA on heart mechanics, which results in deteriorated myocardial contractility before global cardiac function is affected. The findings of this study indicate this rabbit model can be used to elucidate detailed ventricular remodeling capabilities of the heart under different loading conditions such as those occurring in CoA and a wide variety of congenital heart diseases.Acknowledgements
Funding source: NIH R01HL142955 (PI: LaDisa)References
- Wendell et al. J Surg Res. 218:194-201
- Menon et al. Am J Physiol Heart Circ Physiol. 303:H1304-18
- Kwon et al. Pediatr Cardiol. 35:732-740
- Coogan et al. J Thorac Cardiovasc Surg. 145:489-495
- LaDisa et al. J Biomech Eng. 133(9):091008
Figures
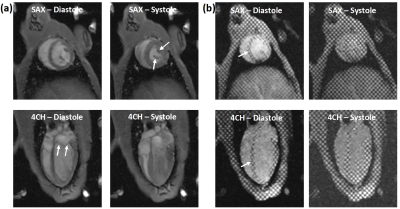
Figure 1. Cine (a) and tagged (b) short-axis (SAX) and 4-chamber (4CH) images
at end-diastole and end-systole acquired with optimal imaging parameters.
Images details, e.g. papillary muscle, valve leaflets, and tagging pattern
(arrows), are clearly visible despite free-breathing acquisition.
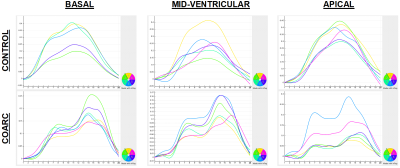
Figure 2. Circumferential strain curves at basal (left), mid-ventricular
(middle), and apical (right) slices in control (top) and CoArc (bottom)
rabbits. The curves show noticeable lower strain values and more heterogeneous
strain pattern in CoArc. Note strain curves appear flipped upside down due to
the use of peripheral triggering, resulting in end-systole happening at early
acquired frames post peripheral triggering.
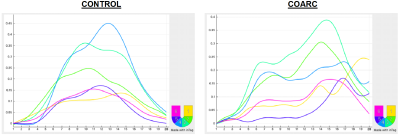
Figure 3. Longitudinal strain curves in control (left) and CoArc (right)
rabbits. The curves show lower strain values and more heterogeneous pattern in
CoArc. Note strain curves appear flipped upside down due to the use of
peripheral triggering, resulting in end-systole happening at early acquired
frames post peripheral triggering.