2182
Global ventricular force-length loops and the quantification of longitudinal and radial contribution to stroke work1Clinical Physiology, Lund University, Lund, Sweden, 2Biomedical Engineering, Lund University, Lund, Sweden, 3Syntach AB, Lund, Sweden, 4Wallenberg Center for Molecular Medicine, Lund University, Lund, Sweden
Synopsis
The concept of global left ventricular force-length loops is introduced as a method for quantifying the contribution to stroke work resulting from longitudinal and radial pumping mechanics, and validated in an animal model. The force-length loops can be derived noninvasively using cardiovascular magnetic resonance and a brachial cuff pressure. We found that longitudinal and radial pumping contributes equally to stroke work in healthy controls and patients with dilated ischemic cardiomyopathy, but that the longitudinal pumping is more energy efficient in delivering stroke volume compared to radial pumping.
Introduction
Left ventricular (LV) shortening can be described by the longitudinal and radial pumping mechanisms. Longitudinal pumping refers to the atrioventricular valve (AV) plane displacement in the apical-basal direction and has been shown to be an independent predictor for major adverse cardiovascular events and mortality1,2, with a contribution of ~60% to LV stroke volume (SV)3,4. The remaining ~40% is accounted for by the radial pumping, which refers to the squeezing inward motion of the epicardium3,4. The directional contribution to stroke work (SW) has however not been investigated previously as it is calculated from pressure-volume (PV) loops where longitudinal and radial function are not reflected.In this study we propose a novel method to calculate SW from ventricular force-length (FL) loop representations of longitudinal and radial pumping. We hypothesize that the sum of the area within these two FL loops equals the PV loop-derived SW. The aim of this study was therefore to develop and experimentally validate such method, and to further explore the directional contributions to SW in healthy controls and patients with dilated ischemic cardiomyopathy.
Methods
A porcine experimental setup consisting of 13 cardiovascular magnetic resonance (CMR) examinations and invasive LV pressure catheterizations was performed. Seven examinations were performed at baseline and 6 at one week post induction of myocardial infarction. Additionally, 12 human healthy controls (29±8 years, EF 59±5%, 8 male) and 14 patients with dilated ischemic cardiomyopathy (69±8 years, EF 32±10%, 13 male) underwent CMR and a noninvasive PV loop quantification based on the time-varying elastance model5.Delineation of the LV endocardial and epicardial borders was performed in short-axis cine images in all slices and phases, and AV-plane displacement was quantified using feature-tracking in long-axis cine images6. Invasive or noninvasive LV pressure over time was synchronized to the LV volume curve measured in the CMR images.
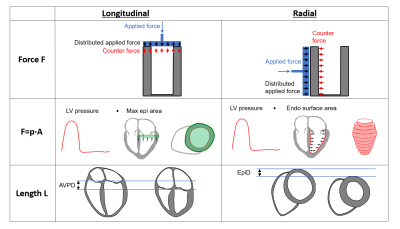
Two FL loops were generated, one longitudinal and one radial (Figure 1). Force (F) was calculated as pressure (P) multiplied with the myocardial surface area (A) in each respective direction, F=P·A. Length was defined as the AV-plane displacement in the longitudinal loop, and as the average change in epicardial radial displacement in the radial loop. Longitudinal and radial contributions to SW were calculated as the area within each respective FL loop. The method was validated by comparing the sum of longitudinal and radial SW with the PV loop-derived SW.
Furthermore, the parameter work per ejected volume (WEV) was calculated as a measure of ventricular energy efficiency in delivering SV, and was defined as the ratio of SW and SV directional contributions as WEVlong=SWlong/SVlong and WEVradial=SWradial/SVradial, respectively. The directional contributions to SV were calculated as previously proposed using AV-plane displacement the end-diastolic basal epicardial cross-sectional area3,4.
Results
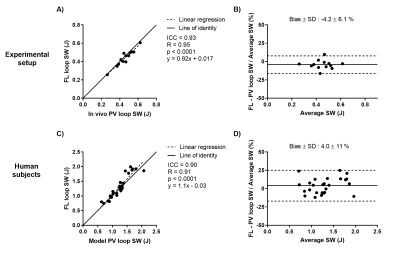
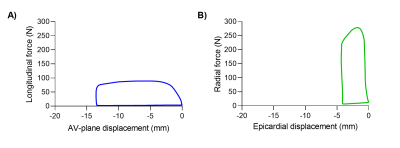
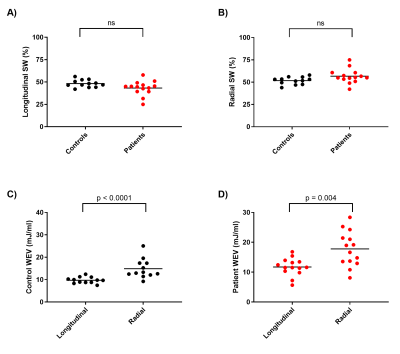
The proposed method was feasible in all subjects and revealed an excellent agreement between FL and PV loop derived SW in the experimental setup and human cohort (Figure 2). An example of two generated FL loops are shown in Figure 3. Longitudinal and radial contribution to SW was approximately equal in swine at baseline (52% vs 48%), swine post myocardial infarction (53% vs 47%), human controls (48% vs 52%), and patients (43% vs 57%), see Figure 4A-B.WEV calculations revealed lower values for longitudinal pumping compared radial in both controls and patients (Figure 4C-D), meaning that longitudinal pumping require less work to eject blood.
Discussion
This study found that longitudinal and radial pumping contribute equally to SW in controls and patients with dilated ischemic cardiomyopathy. The maintained equal contribution to SW in the patients is explained by an increased force due to larger myocardial cross-sectional surface areas in the dilated LV, while length is reduced due to decreased tissue displacements. With longitudinal and radial contributions to SV being ~60% and ~40%, this finding might be surprising until also considering WEV which found longitudinal pumping to be more energy efficient in delivering SV compared to radial pumping.Conclusion
Longitudinal and radial contributions to SW can be calculated from ventricular FL loops, and their sum agrees excellently with the conventional PV loop-derived SW. Longitudinal and radial contribution to SW are approximately equal in controls and dilated ischemic cardiomyopathy patients, but longitudinal pumping is more energy efficient compared to radial pumping.Acknowledgements
No acknowledgement found.References
1. Rangarajan et al. Left ventricular long axis function assessed during cine-cardiovascular magnetic resonance is an independent predictor of adverse cardiac events. J Cardiovasc Magn Reson 18: 35, 2016.
2. Romano et al. Left Ventricular Long-Axis Function Assessed with Cardiac Cine MR Imaging Is an Independent Predictor of All-Cause Mortality in Patients with Reduced Ejection Fraction: A Multicenter Study. Radiology 286: 452–460, 2018.
3. Carlsson et al. Atrioventricular plane displacement is the major contributor to left ventricular pumping in healthy adults, athletes, and patients with dilated cardiomyopathy. AJP Hear Circ Physiol 292: H1452–H1459, 2006.
4. Carlsson et al. The quantitative relationship between longitudinal and radial function in left, right, and total heart pumping in humans. AJP Hear Circ Physiol 293: H636–H644, 2007.
5. Seemann et al. Noninvasive Quantification of Pressure-Volume Loops From Brachial Pressure and Cardiovascular Magnetic Resonance. Circ Cardiovasc Imaging 12: e008493, 2019.
6. Seemann et al. Time-resolved tracking of the atrioventricular plane displacement in Cardiovascular Magnetic Resonance (CMR) images. BMC Med Imaging 17: 19, 2017.
Figures