2180
Left Ventricular Twist and Circumferential Strain from MRI Tagging Assess Early Cardiovascular Disease in Duchenne Muscular Dystrophy1Bioengineering, University of California, Los Angeles, CA, United States, 2Radiology, University of California, Los Angeles, CA, United States, 3Physics and Biology in Medicine IDP, University of California, Los Angeles, CA, United States, 4Radiology, Stanford University, Stanford, CA, United States, 5Biostatistics, University of California, Los Angeles, CA, United States, 6Pediatrics, University of California, Los Angeles, CA, United States
Synopsis
Duchenne Muscular Dystrophy is a common fatal inherited genetic disorder impacting 1:3800 male births, and cardiac failure is the primary source of mortality in this cohort. Decreases in LVEF measured by CINE and fibrosis measured by LGE are late and highly variable outcomes. Herein we demonstrate that peak systolic circumferential strain (Ecc) and Twist measured by MR Tagging provide evidence of earlier changes in cardiac function in a substantial DMD cohort, holding promising applications for patient treatment and the evaluation of novel therapeutics.
Introduction
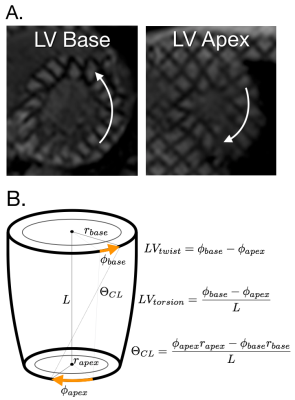
Duchenne Muscular Dystrophy (DMD) is the most common fatal genetic disorder, impacting 1:3,800 male births and ultimately leading to respiratory or cardiac failure during late adolescence [1-2]. The progression of cardiac disease is highly variable in DMD [3-4], and while decreases in left ventricular ejection fraction (LVEF) and presence of cardiac fibrosis as measured by Late Gadolinium Enhancement Positivity (LGE+) from MRI have proven useful, these changes occur late in the disease process and are highly variable [5-8].Earlier biomarkers of cardiac dysfunction are vital for treatment planning and the evaluation of novel therapeutics. Peak systolic mid-wall LV circumferential strain (Ecc) derived from MRI tagging has been reported to effectively distinguish DMD patients from normal volunteers despite no significant differences in LVEF [8-10]. Peak LV twist, torsion, and circumferential-longitudinal shear angle (θ_CL) (Figure 1) measured from MR Tagging may also be earlier biomarkers of cardiac dysfunction in this cohort [11-12].
The objectives of this study were: 1) to characterize a spectrum of functional and rotational LV biomarkers in a cohort of boys with DMD in comparison to normal age-matched controls; and 2) to identify LV biomarkers that detect the onset of cardiomyopathy in the absence of abnormal LVEF or LGE+.
Methods
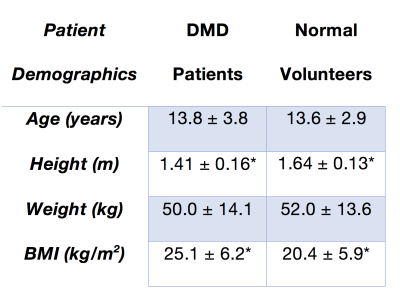
Study Population – with IRB approval, pediatric patients with DMD (N=43, all male, age=13.8±3.8 years) and age-matched healthy volunteers with no history of cardiac disease (N=16, all male, age=13.6±2.9 years). Table 1.MRI Protocol – 1.5T or 3T (Siemens Avanto or Skyra). The exam included: A) breath-held basal, mid-ventricular, and apical LV short-axis grid tagged MRI (resolution = 1.4x1.4x8mm, TE/TRes=2.12/24-48 ms, 11-31 phases, tag spacing=8mm), B) post-contrast CINE imaging with either breath held (N=17) cine bSSFP (matrix = 256x156, resolution = 1.4x1.4 mm, TE/TRes=1.2/28.1-45.1 FA = 40-54º, BW = 800-1300) or free-breathing cine bSSFP [13] (N=26, matrix = 192x120, resolution = 1.4x1.4, TE/TRes=1.2/45.1-64.4 ms, FA = 40º, BW = 930). Healthy volunteers (N = 16) were imaged with an identical free-breathing protocol without contrast, and C) Post contrast conventional breath-held LGE (N=17, resolution: 1.4x1.4x6.0 mm TE/TRes: 2.01/750 ms) or free breathing LGE Imaging (N=26, spatial resolution: 1.4x1.4x8.0mm, TE/TRes: 1.19/904 ms, averages = 8) [14]
Image Analysis - CINE and LGE images were analyzed by clinicians (PR or AP) using commercial software (Circle CVI42, Circle Cardiovascular Imaging Inc.) or Medis (Medis Cardiovascular Imaging). Peak LV mid-wall Lagrangian circumferential strain (Ecc) at the mid-ventricular level and LV basal and apical angular rotation were estimated from tagged MRI (Diagnosoft, Myocardial Solutions). Peak LV twist, torsion, and CL-shear angle (θ_CL) were defined as shown in Figure 1 [15].
Statistical Analysis - Normally distributed data were compared with a two-tailed t-test and a Box-Cox transformation accounted for non-normal data. Holm-Sidak post hoc correction accounted for multiple comparisons. A sub-analysis stratified patients into “LGE(-) DMD” (no scar present) and “LGE(+) DMD” groups. Multiple-regression analysis was used to test for relationships between LVEF and peak mid-wall LV Ecc, twist, torsion, and θ_CL. Significant correlations were defined as a p-value <0.05 compared to the null hypothesis that the data was best correlated with a constant term.
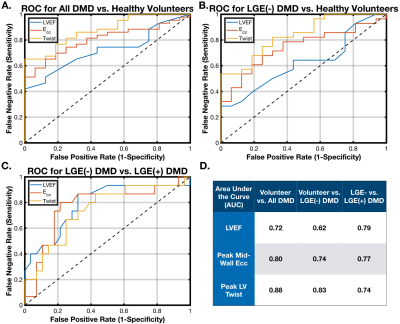
A binomial logistic regression model tested the ability of each measured biomarker to distinguish: 1) healthy volunteers from a DMD population; 2) healthy volunteers from LGE(-) DMD patients; and 3) LGE(-) DMD patients from LGE(+) DMD patients. Results were displayed as receiver-operator curves (ROC), and the area under the curve (AUC) was reported to demonstrate the predictive ability of each biomarker.
Results
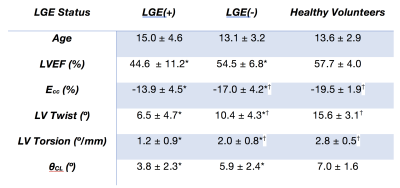
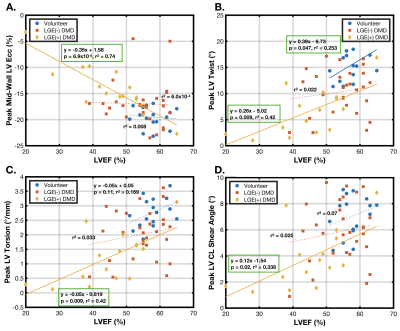
There was a significant difference between patients with DMD and volunteers in LVEF (51.1±9.7% vs. 57.7±4.0%, p<0.01), peak mid-wall Ecc (-15.9±4.5% vs. -19.5±1.9%, p<3.9x10-4), peak LV twist (9.0±4.7° vs. 15.6±3.1°, p<1.1x10-4), peak LV torsion (1.7±0.9°/mm vs. 2.8±0.50°/mm, p<1.1x10-4), and peak LV θ_CL (5.2±2.5° vs. 7.04±3.5°, p<2.1x10-3).Table 2 shows a sub-analysis of these results separated into healthy volunteers, LGE(-) DMD and LGE(+) DMD groups. There was a statistically significant reduction in peak mid-wall Ecc and peak LV torsion between healthy volunteers and LGE negative patients with DMD, but no corresponding significant difference in LVEF. Furthermore, in DMD patients without fibrosis ( LGE(-) ), LVEF was not substantially reduced outside a standard “normal” threshold of < 55%. Notably, there was a further significant and progressive reduction in all cardiac MRI biomarkers between LGE(-) and LGE(+) patients with DMD.
Discussion/Conclusion
Patients with DMD exhibit decreases in peak LV Twist, Torsion, θ_CL, and Ecc that precede decreases in LVEF or the presence of scar as measured by LGE. While all cardiac MRI biomarkers (including LVEF) were effective at distinguishing between the DMD and age-matched healthy population and between LGE(+) and LGE(-) DMD, only peak LV twist, torsion, and Ecc significantly distinguished between the healthy volunteers and the LGE(-) DMD population. Differentiating LGE(-) DMD patients from a population of age-matched healthy volunteers provides important evidence that these cardiac MRI biomarkers highlight early signs of cardiac dysfunction.Both peak LV Twist and Ecc are linked to underlying cardiomyocyte performance, an early “snapshot” of the transition between normal cardiac performance and later DMD cardiomyopathy as measured by LGE(+) and reduced LVEF. This early information is vital in the evaluation of novel therapeutics and in the early treatment of cardiac symptoms in DMD.
Acknowledgements
NIH R01 HL131823 to DBEReferences
1. McNally EM, Kaltman JR, Benson DW, Canter CE, Cripe LH, Duan D, Finder JD, Groh WJ, Hoffman EP and Judge DP. Contemporary cardiac issues in Duchenne muscular dystrophy. Circulation. 2015;131:1590-1598.
2. Weingärtner S, Roujol S, Akçakaya M, Basha TA and Nezafat R. Free‐breathing multislice native myocardial T1 mapping using the slice‐interleaved T1 (STONE) sequence. Magnetic resonance in medicine. 2015;74:115-124.
3. Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C and Pandya S. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. The Lancet Neurology. 2010;9:177-189.
4. Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C and Pandya S. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. The Lancet Neurology. 2010;9:77-93.
5. Tandon A, Villa CR, Hor KN, Jefferies JL, Gao Z, Towbin JA, Wong BL, Mazur W, Fleck RJ and Sticka JJ. Myocardial fibrosis burden predicts left ventricular ejection fraction and is associated with age and steroid treatment duration in Duchenne muscular dystrophy. Journal of the American Heart Association. 2015;4:e001338.
6. Silva MC, Meira ZMA, Giannetti JG, da Silva MM, Campos AFO, de Melo Barbosa M, Starling Filho GM, de Aguiar Ferreira R, Zatz M and Rochitte CE. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. Journal of the American College of Cardiology. 2007;49:1874-1879.
7. Puchalski MD, Williams RV, Askovich B, Sower CT, Hor KH, Su JT, Pack N, Dibella E and Gottliebson WM. Late gadolinium enhancement: precursor to cardiomyopathy in Duchenne muscular dystrophy? The international journal of cardiovascular imaging. 2009;25:57-63.
8. Hor KN, Wansapura J, Markham LW, Mazur W, Cripe LH, Fleck R, Benson DW and Gottliebson WM. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy. Journal of the American College of Cardiology. 2009;53:1204-1210.
9. Ashford M, Liu W, Lin S, Abraszewski P, Caruthers S, Connolly A, Yu X and Wickline SA. Occult cardiac contractile dysfunction in dystrophin-deficient children revealed by cardiac magnetic resonance strain imaging. Circulation. 2005;112:2462-2467.
10. Hagenbuch SC, Gottliebson WM, Wansapura J, Mazur W, Fleck R, Benson DW and Hor KN. Detection of progressive cardiac dysfunction by serial evaluation of circumferential strain in patients with Duchenne muscular dystrophy. The American journal of cardiology. 2010;105:1451-1455.
11. Reyhan ML, Wang Z, Kim HJ, Halnon NJ, Finn JP and Ennis DB. Effect of free‐breathing on left ventricular rotational mechanics in healthy subjects and patients with duchenne muscular dystrophy. Magnetic resonance in medicine. 2017;77:864-869.
12. Reyhan M, Wang Z, Li M, Kim HJ, Gupta H, Lloyd SG, Dell'Italia LJ, Denney T and Ennis DB. Left ventricular twist and shear in patients with primary mitral regurgitation. Journal of Magnetic Resonance Imaging. 2015;42:400-406.
13. Kellman P, Chefd'hotel C, Lorenz CH, Mancini C, Arai AE and McVeigh ER. Fully automatic, retrospective enhancement of real‐time acquired cardiac cine MR images using image‐based navigators and respiratory motion‐corrected averaging. Magnetic resonance in medicine. 2008;59:771-778.
14. Kellman P, Larson AC, Hsu LY, Chung YC, Simonetti OP, McVeigh ER and Arai AE. Motion‐corrected free‐breathing delayed enhancement imaging of myocardial infarction. Magnetic resonance in medicine. 2005;53:194-200.
15. Young AA and Cowan BR. Evaluation of left ventricular torsion by cardiovascular magnetic resonance. Journal of Cardiovascular magnetic resonance. 2012;14:49.
Figures