2174
Optimized ultra-high-field cardiac magnetic resonance imaging
El-Sayed H Ibrahim1, V Emre Arpinar1, L Tugan Muftuler1, Andrew Nencka1, and Kevin Koch1
1Medical College of Wisconsin, Milwaukee, WI, United States
1Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Cardiac functional MRI has been established in clinical practice on 1.5T and 3T scanners; nevertheless, the capabilities of ultra-high field (UHF) MRI have not been fully exploited, which are expected to significantly improve image quality and provide information not obtainable at lower-field MRI. Despite its advantages, UHF MRI is challenging due to a number of technical issues, especially B1 and B0 inhomogeneities. In this study, we provide preliminary results of using a multi-channel transceiver modular coil and a dielectric pad towards optimizing UHF cardiac functional MRI by improving image quality and minimizing imaging artifacts.
Introduction
Cardiac functional MRI has been established in clinical practice on 1.5T and 3T scanners. Nevertheless, the capabilities of ultra-high field (UHF) MRI have not been fully exploited, which are expected to significantly improve image quality and provide more information that allow for better understanding of cardiac function in both normal and different pathophysiological conditions.1 UHF MRI incorporates several advantages as well as some limitations. As signal-to-noise ratio (SNR) is proportional to magnetic field strength, 7T MRI provides ~2.3 times SNR compared to 3T imaging. Enhanced SNR can be traded for improved image quality, higher temporal or spatial resolution, or shorter scan time. Despite its advantages, UHF MRI is challenging due to a number of technical issues.1 First, the applied radiofrequency (RF) fields at such high frequencies result in short wavelength (~12 cm) of the RF fields inside the tissue and increased dielectric effects that can induce destructive and constructive interferences and result in distorted RF fields and non-uniform effective flip angle distribution with observed shading and signal drop-off. Imaging at UHF also results in increased B0 inhomogeneity due to magnetic susceptibility effects. In this study, we provide preliminary results of using a multi-channel transceiver modular coil and a dielectric pad towards optimizing MRI cardiac functional imaging at 7T and improving image quality while alleviating artifacts associated with UHF imaging.Methods
Ten healthy subjects were scanned on a 7T GE MRI scanner using a 32-channel transceiver coil. The modular coil array consists of 8 independent blocks, where each block contains 4 transceiver elements, whose phase settings were optimized based on simulations for a multi-oblique plane mimicking a standard cardiac view.2 The effect of the imaging flip angle on image quality was assessed in vivo using a gradient-echo cine sequence. Image acquisition was repeated with flip angles ranging from 1° to 120°. Forward simulation was conducted on the resulting images to generate an estimate of the B1 transmission frequency field in the imaged slice using actual imaging flip-angle (AIF) B1 mapping.3 Both short-axis (SAX) and long-axis (LAX) cardiac cine images were acquired in the scanned subjects using optimal imaging parameters: fast spoiled gradient echo sequence, TR=8ms, TE=4ms, flip angle=60°, matrix=256x256, FOV=380x380 mm2, slice thickness=8mm, acquisition bandwidth=244Hz/pixel, #averages =1, #cardiac phases =25, and SAR=0.095W/kg. Images with higher spatial and temporal resolutions were also acquired to assess capabilities of UHF cardiac functional imaging. The effect of using a dielectric pad to improve B1 homogeneity was investigated in six subjects (three normal-weight and three over-weight) with the pad placed at different positions close to the imaged region-of-interest.Results
With proper settings and imaging parameters optimization, we were able to achieve sufficient RF penetration and B1 homogeneity across the heart, which allowed for successfully scanning all subjects with decent image quality. The ECG signal was reliable in all in vivo scans and image acquisition was correctly triggered with the R-wave, despite elevated T-wave in most scans. Figure-1 shows results from an in-vivo scan to estimate the B1 field. The figure shows variable signal intensity and regions of signal loss across the acquired slice, where the signal intensity profile changes with the imaging flip angle. The estimated B1 and proton density distribution maps reflect the observed signal intensity inhomogeneity in the imaged slice. The resulting R2 map shows excellent data fitting, as shown for certain pixels located in the imaged slices. Adding a dielectric pad close to the imaged slice could help improve B1 homogeneity (Figure-2). Moving the pad around the thorax area affects the presence and location of the signal nulling regions, where in most cases placing the pad anteriorly improved signal homogeneity in normal-weight subjects, while subjects with larger body habitus showed adequate signal homogeneity even without using the dielectric pad. Exploiting the capabilities of UHF imaging allowed for achieving high-spatial resolution of 0.75x0.75x2 mm3, which is ~16 times better than conventional cine imaging at 1.5T (Figure-3). Such high spatial resolution allows for visualizing detailed anatomical features without sacrificing SNR as in low-field imaging. Alternatively, higher temporal resolution of ~20 ms (50 cardiac phases) could be achieved to reveal subtle tissue motion pattern.Discussion
While the majority of UHF MRI studies have focused on brain and orthopedic applications, recent improvements in multi-channel coil development would open the door for cardiac imaging to benefit from UHF capabilities.2,4 The transceiver modular coil used in this study allowed for obtaining high-quality in vivo images with high resolution and adequate myocardium-to-blood contrast, while minimizing B1 non-uniformity and shading effects. It has been previously shown that actual flip angle varies by as much as 50% from the nominal flip angle across the heart during high-field imaging.5 Adjusting the imaging flip angle and adding a dielectric pad to the imaged region-of-interest, especially when imaging subjects with small body habitus, could help improve B1 homogeneity and reduce signal nulling resulting from standing-wave effects.Conclusion
In conclusion, through proper scan settings and imaging parameter optimization, 7T cardiac MRI would allow for improved cardiac functional imaging. This is expected to open the door for more cardiac applications of UHF MRI and potential adoption in clinical practice in the near future.Acknowledgements
Funding from Daniel M. Soref Charitable Trust, MCW, USA.References
- Niendorf T et al. NMR Biomed 2016;29(9):1173-97.
- Graessl A et al. Magn Reson Med 2014;72(1):276-90.
- Yarnykh VL et al. Magn Reson Med 2007;57(1):192-200.
- Thalhammer C et al. J Magn Reson Imaging 2012;36(4):847-57.
- Suttie JJ et al. NMR Biomed 2012;25(1):27-34.
Figures
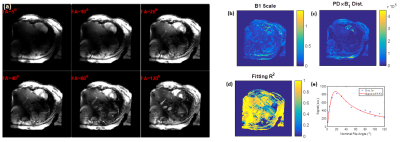
Figure 1. Estimation of the B1 transmission field in a volunteer scan. (a)
Gradient-echo magnitude images with different excitation flip angles. (b) B1
scale factor map. (c) Proton density distribution map. (d) Goodness-of-fit map.
(e) Example of signal intensity measurements at different flip angles (dots) and
the fitting curve (red line) for a certain pixel in the heart.
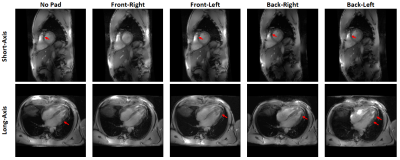
Figure 2. Short-axis and
long-axis slices acquired with dielectric pad placed at different locations
around the thorax area. Note how signal intensity drops appear at different
regions (arrows) based on pad location. In the case of this subject, placing
the pad at right anterior position resulted in most uniform signal intensity.
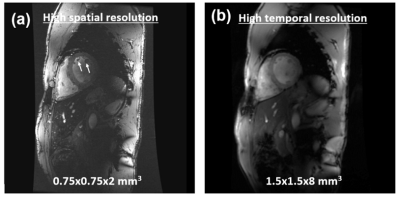
Figure 3. 16-fold increase in spatial resolution can be achieved at 7T. (a)
High-resolution image illustrates more anatomical details (arrows) and results
in accurate cardiac function measurements compared to conventional resolution
as in (b). (b) Alternatively, high temporal resolution (~ 20ms) can be achieved
while using conventional spatial resolution.