2168
MR detects improvement in cardiac function and fibrosis after micro-dystrophin treatment in dystrophic mice1Radiology, University of Washington, Seattle, WA, United States, 2Neurology, University of Washington, Seattle, WA, United States, 3Medicine, University of Washington, Seattle, WA, United States
Synopsis
Cardiomyopathy is an inevitable fate for patients with Duchenne muscular dystrophy (DMD) and is one of the major causes of mortality. Cardiovascular magnetic resonance (CMR) is increasingly being performed at very high magnetic field strength for small animal models of muscular dystrophy. The mdx mouse model is one of the most commonly used animal models for DMD. Recombinant adeno-associated viral vector-mediated gene transfer represents a promising approach for DMD. The aim of this study was to elucidate the functional impact of micro-dystrophin on cardiomyopathy in mdx mice using CMR as a non-invasive biomarker.
Introduction
Duchenne muscular dystrophy (DMD), an X-linked autosomal inherited disease is characterized by the absence of functional dystrophin leading to cardiomyopathy [1, 2]. The mdx mouse model is one of the most commonly used animal models for DMD. Cardiac magnetic resonance (CMR) has been extensively used to identify changes in systolic volumes and ejection fraction (EF) in the right and left ventricles of muscular dystrophy patients [3, 4]. Furthermore, late gadolinium-enhanced (LGE) MR imaging has been used to identify fibrotic and inflamed tissue in the myocardium. In the mdx mouse, standard measures of left ventricular function, including ejection fraction, end-systolic volume and wall thickening, do not become abnormal until 9 to 11 months of age [5, 6]. Adeno-associated viral (AAV) vectors are well characterized as gene-therapy tools with the capacity for systemic delivery [7, 8]. The purpose of this study was to monitor the treatment effects of AAV vector-mediated micro-dystrophin (μDys) gene therapy using CMR as a non-invasive biomarker.Methods
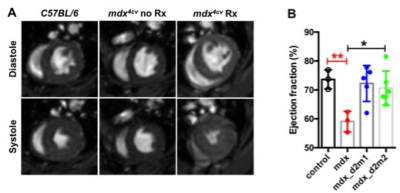
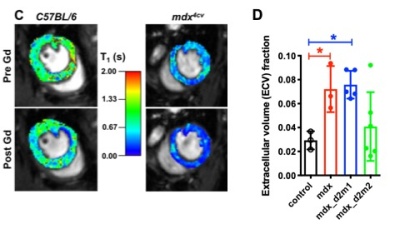
Male wild type C57BL/6J (ctrl; n=3) and mdx4cv (mdx; n=14) mice were utilized for the study. mdx (n=11) mice were treated with two different of AAV vectors, rAAV6-CK8-µDys1 (µDys1; n=5) and rAAV6-CK8-µDys2 (μDys 2; n=6) at 2 weeks of age with a dose of 4 x 1014 vg/kg. In-vivo skeletal MRI was performed on a 14 tesla (T) NMR spectrometer (Bruker Biospin, Billerica, MA) at 68 weeks of age. Mice were anesthetized using 3% isoflurane and maintained at 1% isoflurane and a mixture of air and oxygen at 3:1 ratio by using a vaporizer. The vertical bore of the magnet was maintained at 30oC to maintain thermo-neutrality of the animal and respiratory rate was monitored and maintained at 50-70 breaths/min by adjusting the anesthetic concentration. The MR system was interfaced to a console running ParaVision software 5.1 including the IntraGate software for sequence acquisition and reconstruction. Single slice coronal and sagittal long-axis scans (TR/TE = 5/2.2 ms) were acquired to view apex and mitral valve planes. These long-axis scans were used to acquire 5 short-axis (TR/TE = 5/2.2 ms) scans, which were then used to measure left ventricular function. In order to obtain the global parameters of the entire heart, the volume for each frame was calculated as the sum of the area of interest in each slice multiplied by the slice thickness. Ejection fraction (EF%) was calculated from the blood volume, determined in the end-systolic and end-diastolic phase. Furthermore, myocardial extracellular volume (ECV) was measured as the percent of tissue comprised of extracellular space [9]. ECV was calculated using T1-maps acquired pre and post Gadolinium (Gd) contrast using the following parameters (TR/TE = 5/2.2 ms, flip angle = 2o, 5o, 10o, 20o, 40o, 60o) and calibrated by blood hematocrit.Results and Discussion
Significant differences were noted between EF (%) of ctrl and mdx mice (73.63 ± 3.33 % vs 59.07 ± 3.54 %, p<0.01). Treatment with AAV-μDys improved EF in dystrophic myocardium (mdx; 59.07 ± 3.54 %, mdx-d2m1; 72.24 ± 6.22 % and mdx-d2m2; 70.66 ± 5.91 %, p<0.05). In the mdx mouse EF and other myocardium functional measures become abnormal after 11 months of age. Similarly, we have demonstrated a decrease in EF in mdx mice at 17 months of age. One of the reasons for a decrease in EF is accumulation of collagenous/non-contractile tissue in the dystrophic myocardium. Furthermore, we have quantified the amount of collagen fraction (ECV) using the T1 weighted Gd contrast. The amount of ECV was significantly higher in mdx mice compared to ctrl mice (0.07 ± 0.02 vs 0.03 ± 0.007). Finally, treatment with AAV-μDys (mdx-d2m2) decreased ECV compared to mdx untreated myocardium (0.04 ± 0.03 vs 0.07 ± 0.02). These observations and measurements could be further explored and validated with comparisons to histological measurements for a more nuanced understanding of the cellular change.Acknowledgements
This work is supported by NIH R01CA188654, NIH 2P50 AR065139, NIH R01 AR40864-27, and MDA 312455. We would like to thank Yasser Nazari for his assistance with data acquisition.References
1. Fayssoil, A., et al., Cardiomyopathy in Duchenne muscular dystrophy: pathogenesis and therapeutics. Heart Failure Reviews, 2010. 15(1): p. 103-107.
2. Spurney, C.F., Cardiomyopathy of Duchenne muscular dystrophy: current understanding and future directions. Muscle & Nerve, 2011. 44(1): p. 8-19.
3. Hoerr, V., et al., Cardiac-respiratory self-gated cine ultra-short echo time (UTE) cardiovascular magnetic resonance for assessment of functional cardiac parameters at high magnetic fields. J Cardiovasc Magn Reson, 2013. 15: p. 59.
4. Bun, S.S., et al., Value of in vivo T2 measurement for myocardial fibrosis assessment in diabetic mice at 11.75 T. Invest Radiol, 2012. 47(5): p. 319-23.
5. Quinlan, J.G., et al., Evolution of the mdx mouse cardiomyopathy: physiological and morphological findings. Neuromuscular Disorders, 2004. 14(8-9): p. 491-496.
6. Zhang, W., et al., Abnormal cardiac morphology, function and energy metabolism in the dystrophic mdx mouse: An MRI and MRS study. Journal of Molecular and Cellular Cardiology, 2008. 45(6): p. 754-760.
7. Gregorevic, P., et al., Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med, 2004. 10(8): p. 828-34.
8. Ramos JN, Hollinger K, Bengtsson NE, Allen JM, Hauschka SD and Chamberlain JS: Development of novel micro-dystrophins with enhanced functionality. Mol Ther 2019; 27:623-635.
9. Kellman, P., et al., Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson, 2012. 14: p. 63.
Figures