2160
Comparison of Non-Contrast Enhanced Dynamic MRA Techniques: 4D-SNAP vs 4D-TRANCE1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2Neusoft Medical Systems Co., Ltd., Shanghai, China, 3Vascular Imaging Laboratory, Department of Radiology, University of Washington, Seattle, WA, United States
Synopsis
Simultaneous Non-contrast Angiography and intraPlaque imaging (SNAP), a non-contrast enhanced MRA technique, shows great potentials for either static artery imaging or 4D time-resolved artery imaging. Arterial spin labeling (ASL) based imaging techniques can also be used for dynamic MRA, e.g., 4D time-resolved angiography non-contrast enhanced (4D-TRANCE). Both of them are compatible with parallel imaging thus practical for clinical usage. But they are based on different principles and their performance comparison is desired. This study aims to compare 4D-SNAP and 4D-TRANCE. Results show 4D-SNAP provides 4D dynamic MRA with higher SNR and fewer artifacts than 4D-TRANCE using either TFE or TFE-EPI.
Introduction
Simultaneous Non-contrast Angiography and intraPlaque imaging (SNAP) as a non-contrast enhanced (NCE) MRA technique was originally developed for carotid artery imaging 1. Recent studies of intracranial SNAP-MRA showed that it could provide similar visualization and stenosis detection accuracy in main arteries when compared with TOF-MRA 2-5. Furthermore, SNAP-MRA was found to provide significantly better small arteries delineation than TOF-MRA. With optimization, it was also extended to 4D MRA for dynamic intracranial artery imaging and showed high potentials for time-resolved MRA 6. Traditionally, the principle of arterial spin labeling (ASL) has been used for dynamic MRA 7-13, by changing the post-labeling-delay (PLD) time gradually. This study aims to compare 4D-SNAP and 4D time-resolved angiography non-contrast enhanced (4D-TRANCE) technique, which is an ASL-based 4D MRA method on the Philips scanner.Methods
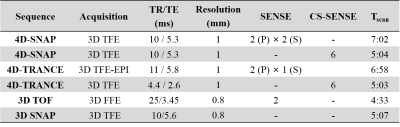
Imaging was performed on a Philips 3.0T Ingenia scanner (Philips Healthcare, Best, The Netherlands) using a 32-channel coil. Two volunteers were recruited with institutional review board approval and written consents were obtained before the study.The 1st experiment was conducted to find the optimal imaging parameters for the two methods. Both 4D-SNAP and 4D-TRANCE, using either SENSE or compressed sensing SENSE (CS-SENSE) 14, were attempted (Table 1). Specifically, TIs and the flip angles used in SNAP were selected based on Table 1 in Reference 6. In 4D-TRANCE, either 3D turbo field echo (TFE) or 3D TFE-EPI was used according to the compatibility of undersampling acceleration. Imaging parameters used: FOV = 160×160×60 mm3. TFE-factor for 4D-TRANCE with 3D TFE was set to 23 to match the scan time of 4D-SNAP with CS-SENSE acceleration, while for 4D-TRANCE using 3D TFE-EPI, TFE factor = 11 and EPI factor = 5. Dynamic images with 8 phases were acquired. In 4D-SNAP, the first TI = 230 ms and interval = 30 ms, while for 4D-TRANCE, the first PLD = 200 ms and interval = 150 ms.
The 2nd experiment in the 2nd volunteer was a validation and reproducibility test. Only 4D-SNAP with CS-SENSE=6 and 4D-TRANCE using 3D TFE-EPI with SENSE=2 were used (Table 1).
In both experiments, 3D TOF with flip angle=20° was acquired as the reference of the intracranial arterial tree. In addition, a static 3D SNAP with TI=500ms and no parallel imaging was acquire as another reference (Table 1). Both FOVs were the same as 4D MRA.
After phase-sensitive reconstruction, the SNAP-MRA images were generated by only displaying the negative signals. Maximum intensity projection (MIP) was conducted. To generate 4D-SNAP, the SNAP images with different TIs were normalized first, then all images were sorted by order of TI.
Results and Discussion
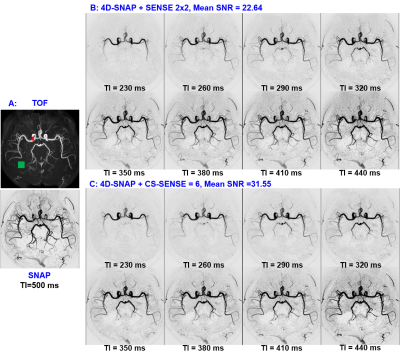
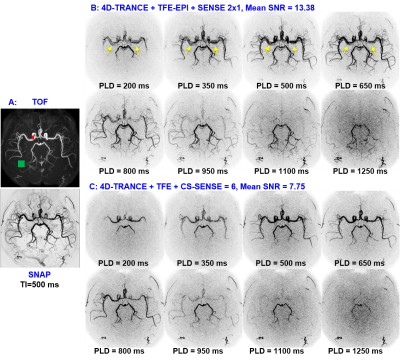
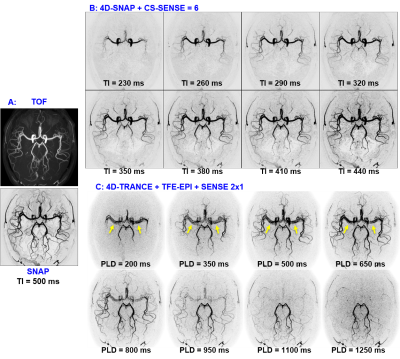
Figs. 1 and 2 show the results for experiment 1. Both 4D-SNAP using different accelerating strategies (Figs. 1B and C) provide satisfactory image quality. In contrast, 4D-TRANCE shows visually higher noise levels than 4D-SNAP (Fig. 2). Additionally, pulsation artifacts (arrows) can be seen in 4D-TRANCE using TFE-EPI. Another observation is that the blood vessels fade in the long PLD images when compared to 4D-SNAP. The noise level in Fig. 2C is higher than that in Fig. 2B. SNR analysis shows that 4D-TRANCE has intrinsically lower SNR than 4D SNAP-MRA.Fig. 3 shows the results for the 2nd experiment. Although the SNRs in 4D-SNAP are lower than the references and some branch arteries are missing, the images can reliably display the blood expanse clearly with acceptable image quality. The pulsation artifacts and the blood signal decay in the long PLD images keep the same in 4D-TRANCE.
The pulsation artifacts should be reduced with ECG gating, which, however, increases acquisition time. The PLD times can be squeezed in a small range to match the similar phases as 4D-SNAP. But the acceleration capability and artifacts in 4D-TRANC+TFE-EPI limit its usage. The SNR in 4D-TRANCE+TFE is low for dynamic MRA, leading to poor visualization of peripheral arteries.
Conclusion
4D-SNAP with better acceleration capability provides dynamic images with higher SNR and fewer artifacts than 4D-TRANCE using either TFE or TFE-EPI.Acknowledgements
No acknowledgement found.References
1. Wang J, Börnert P, Zhao H, et al. Simultaneous noncontrast angiography and intraPlaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magnetic Resonance in Medicine 2013;69(2):337-345.
2. Wang J, Zhao X, Boernert P, Yuan C. Simultaneous intracranial angiography and intraplaque hemorrhage imaging using SNAP. Journal of Cardiovascular Magnetic Resonance 2013;15(Suppl 1).
3. Wang J, Zhao X, Yamada K, et al. High-risk mid-cerebral artery atherosclerotic disease detection using Simultaneous Non-contrast Angiography and intraPlaque khemorrhage (SNAP) imaging. Proceedings of the International Society of Magnetic Resonance in Medicine. Salt Lake City, UT, USA, 2013. p1129.
4. Li Q, Wang J, Chen H, et al. Characterization of Craniocervical Artery Dissection by Simultaneous MR Noncontrast Angiography and Intraplaque Hemorrhage Imaging at 3T. American Journal of Neuroradiology 2015.
5. Wang J, Guan M, Yamada K, et al. In vivo validation of simultaneous non-Contrast angiography and intraPlaque hemorrhage (SNAP) magnetic resonance angiography: an intracranial artery study. PloS one 2016;11(2):e0149130.
6. Xiong Y, Zhang Z, He L, et al. Intracranial simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) MRA: Analyzation, optimization, and extension for dynamic MRA. Magnetic resonance in medicine 2019;82(5):1646-1659.
7. Yu S, Yan L, Yao Y, et al. Noncontrast dynamic MRA in intracranial arteriovenous malformation (AVM): comparison with time of flight (TOF) and digital subtraction angiography (DSA). Magnetic Resonance Imaging 2012;30(6):869-877.
8. Song HK, Yan L, Smith RX, et al. Noncontrast enhanced four‐dimensional dynamic MRA with golden angle radial acquisition and K‐space weighted image contrast (KWIC) reconstruction. Magnetic Resonance in Medicine 2014;72(6):1541-1551.
9. Yan L, Liu CY, Smith RX, et al. Assessing intracranial vascular compliance using dynamic arterial spin labeling. Neuroimage 2016;124:433-441.
10. Cong F, Zhuo Y, Yu S, et al. Noncontrast‐enhanced time‐resolved 4D dynamic intracranial MR angiography at 7T: A feasibility study. Journal of Magnetic Resonance Imaging 2018;48(1):111-120.
11. van Osch MJ, Teeuwisse WM, Chen Z, Suzuki Y, Helle M, Schmid S. Advances in arterial spin labelling MRI methods for measuring perfusion and collateral flow. Journal of Cerebral Blood Flow & Metabolism 2018;38(9):1461-1480.
12. Zhou Z, Han F, Yu S, et al. Accelerated noncontrast‐enhanced 4‐dimensional intracranial MR angiography using golden‐angle stack‐of‐stars trajectory and compressed sensing with magnitude subtraction. Magnetic Resonance in Medicine 2018;79(2):867-878.
13. Obara M, Togao O, Beck GM, et al. Non-contrast enhanced 4D intracranial MR angiography based on pseudo-continuous arterial spin labeling with the keyhole and view-sharing technique. Magnetic Resonance in Medicine 2018;80(2):719-725.
14. Liang D, Liu B, Wang J, Ying L. Accelerating SENSE using compressed sensing. Magnetic Resonance in Medicine 2009;62(6):1574-1584.
Figures