2158
Feasibility of Simultaneous Non-contrast Angiography and intraPlaque hemorrhage Imaging (SNAP) in Abdominopelvic Angiography1Biomedical Engineering, Tsinghua University, Beijing, China, 2Philips Healthcare, Beijing, China
Synopsis
Non-contrast enhanced MRA of abdominopelvic arteries within a large field of view is very challenging due to rapid blood flow and variant flow speed among different subjects. Simultaneous Non-contrast Angiography and intraPlaque imaging (SNAP), a non-contrast enhanced MRA technique, provides reliable imaging for the carotid and intracranial arteries. In this study, we sought to explore the feasibility of SNAP with compressed sensing acceleration in abdominopelvic arteries imaging. The complementary information provided by different scans with different velocity sensitivities can be combined to obtain reliable abdominopelvic MRA with a large coverage.
Introduction
Non-contrast enhanced MRA of abdominopelvic arteries within a large field of view is very challenging due to rapid blood flow and variant flow speed among different subjects. Arterial spin labeling (ASL) based MRA techniques have already been attempted in the abdominopelvic arteries, but the reliability is not guaranteed1.Simultaneous Non-contrast Angiography and intraPlaque imaging (SNAP), a different non-contrast enhanced MRA technique with ASL, provides reliable imaging for the carotid and intracranial arteries2-6. However, the feasibility of SNAP MRA in the abdomen with a large FOV has not examined and thus an investigation is desired. In this study, we sought to determine the feasibility of SNAP with compressed sensing acceleration in renal and external iliac arteries imaging.
Methods
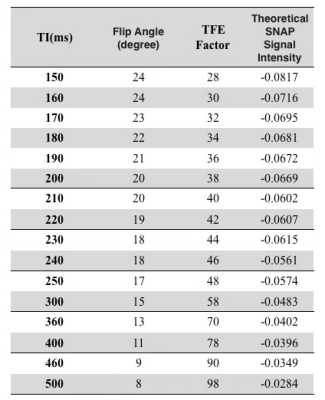
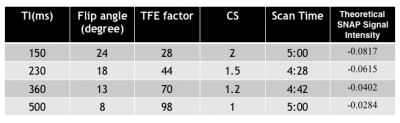
Previously the imaging parameters for SNAP were optimized for either the carotid or intracranial artery imaging2,6. So our first step in this study was to optimize the parameters through Bloch simulation using the abdomen relaxation times. New TI and flip angle combinations covering different flow velocities were obtained for optimal contrast and high SNR (See Table 1).Then in vivo images were acquired on a Philips 3.0T Ingenia CX MR scanner (Philips Healthcare, Best, The Netherlands) using a 16 channel phased array coil. Four volunteers (23.75 ± 5.75 years, 2 males) were recruited in this IRB approved study and written informed consent was obtained from each subject. As the blood flow speed is very different among different subjects for abdominopelvic artery imaging, instead of measuring speed before each SNAP scan, we conducted 4 SNAP scans, each of which is sensitive to a different flow velocity. Different velocity sensitivity needs to use different TI and flip angle combinations, where were obtained in the first step. Turbo field echo (TFE) factor in 3D SNAP was changed accordingly as well according to the previous study, 3D SNAP images were acquired coronally with the following common parameters: FOV = 270x360x100 mm3, TR/TE = 8.9/4.9 ms, resolution 1.5x1.5x1.5 mm3. For different scans, since the TFE factor was not the same, different compressed sensing (CS) acceleration factors were used so the scan time for different scans was kept around 5 minutes. Detailed scan parameters for 4 scans are shown in Table 2.
After phase sensitive reconstruction for SNAP, the phase corrected real images were used to generate a black blood MRA using minimum intensity projection on the scanner. As different scans provide complementary MRA information, they were combined to generate vessel images extending in a large FOV.
Results
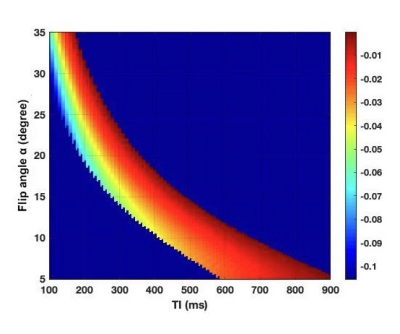
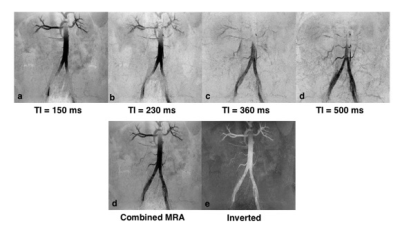
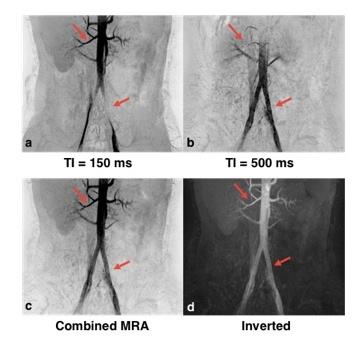
The result of imaging parameter optimization is shown in Figure 1, which represents the theoretical blood signal intensity under different TIs and flip angles. After discarding the lower‐left region with negative background signal and the upper‐right region with positive blood signal, the hot zone reveals the range of TIs and flip angles that satisfy the signal condition. The figure shows that the absolute value of blood signal intensity increases as TI or flip angle decreases. The MRA images using the optimized TIs, flip angles and the corresponding TFE factors for one subject are shown in Figure 2. The renal arteries and the abdominal aorta are well delineated in the image using TI = 150 ms while the iliac arteries are better viewed using longer TIs. The MRAs using TI = 150 ms and TI = 500 ms were combined and smoothed on the scanner to obtain the image shown in Figures 2d and 2e, which shows more arteries than each separate MRA. The combined black blood MRA using TI = 150 ms and TI = 500 ms from another subject are shown in Figure 3. The missing parts of the arteries for each separate MRA are recovered in the combined MRA (red arrows).Discussion and conclusion
In this study, we investigated the feasibility of SNAP for abdominopelvic MRA with a large coronal FOV. The complementary information provided by different scans with different velocity sensitivities can be combined to obtain reliable abdominopelvic MRA with a large coverage. Further systematic clinical studies are warranted to clarify its diagnostic ability.Acknowledgements
No acknowledgement found.References
1. Atanasova I, Kim D, Lim R, et al. Noncontrast MR Angiography for Comprehensive Assessment of Abdominopelvic Arteries using Quadruple Inversion-Recovery Preconditioning and 3D balanced Steady-State Free Precession Imaging. Journal of Magnetic Resonance Imaging, 2011; 33(6):1430–1439.
2. Wang J, Börnert P, Zhao H, et al. Simultaneous noncontrast angiography and intraPlaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magnetic Resonance in Medicine 2013;69(2):337-345.
3. Wang J, Zhao X, Boernert P, Yuan C. Simultaneous intracranial angiography and intraplaque hemorrhage imaging using SNAP. Journal of Cardiovascular Magnetic Resonance 2013;15(Suppl 1).
4. Wang J, Zhao X, Yamada K, et al. High-risk mid-cerebral artery atherosclerotic disease detection using Simultaneous Non-contrast Angiography and intraPlaque khemorrhage (SNAP) imaging. Proceedings of the International Society of Magnetic Resonance in Medicine. Salt Lake City, UT, USA, 2013. p1129.
5. Wang J, Guan M, Yamada K, et al. In vivo validation of simultaneous non-Contrast angiography and intraPlaque hemorrhage (SNAP) magnetic resonance angiography: an intracranial artery study. PloS one 2016;11(2):e0149130.
6. Xiong Y, Zhang Z, He L, et al. Intracranial simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) MRA: Analyzation, optimization, and extension for dynamic MRA. Magnetic resonance in medicine 2019;82(5):1646-1659.
Figures