2156
Optimized Stack-of-Stars QISS MR Angiography for Near-Isotropic Non-Contrast Evaluation of the Renal and Peripheral Arteries1Radiology, NorthShore University HealthSystem, Evanston, IL, United States, 2Radiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States, 3Siemens Medical Solutions USA, Chicago, IL, United States, 4Pritzker School of Medicine, University of Chicago, Chicago, IL, United States
Synopsis
Quiescent-interval slice-selective (QISS) MRA has become established as a simple “push button” nonenhanced alternative to CTA and contrast-enhanced MRA. However, a fundamental drawback of QISS as currently implemented is that it uses a 2D acquisition strategy. The inability to acquire very thin slices along with the non-rectangular slice profile predisposes QISS to artifacts from partial volume averaging and blurring of multiplanar reconstructions. We therefore implemented and optimized a novel thin-slab stack-of-stars QISS (tsSOS-QISS) technique to provide near-isotropic high spatial resolution that rivals CTA, while maintaining the excellent arterial conspicuity, motion insensitivity and breath-hold capability of 2D QISS.
Introduction
Physicians rely primarily on CT angiography (CTA) for the non-invasive evaluation of peripheral artery disease (PAD) in the lower extremities prior to revascularization. For patients with severely impaired renal function who cannot tolerate iodinated or gadolinium-based contrast agents, non-contrast MRA techniques such as quiescent-interval slice-selective (QISS), subtractive fast spin-echo, and velocity-selective 3D MRA provide potentially useful imaging options.1 While QISS is now commercially available and has demonstrated diagnostic accuracy that is competitive with CTA2, it has several well-recognized drawbacks, the most significant of which is the use of a 2D acquisition strategy. The typical QISS slice thickness of 2 to 3-mm is substantially worse than the 1-mm slice thickness provided by peripheral CTA, resulting in partial volume averaging. Moreover, the slice profile is highly non-rectangular, resulting in loss of detail on multiplanar reconstructions. In order to overcome these limitations, a prototype 3D thin-slab stack-of-stars QISS (tsSOS-QISS) technique was recently described.3 As originally implemented, the tsSOS-QISS technique was relatively inefficient with the potential for flow-related saturation and venetian blind artifacts (VBA). In this study, we optimized the technique so as to overcome these limitations and provide a robust alternative to existing 2D QISS implementations.Methods
We performed an IRB-approved, prospective study on healthy volunteers and patients with PAD after obtaining informed, written consent. Scans were performed on a clinical 1.5T scanner (MAGNETOM Avanto Dot, Siemens Healthcare, Erlangen, Germany). 2D QISS was typically acquired using 10 contiguous stations with 40 3mm-thick slices per station. For tsSOS-QISS of the peripheral arteries, a series of thin overlapping 3D slabs were acquired using a balanced steady-state free precession (bSSFP) readout, whereas a single slab was used for breath-hold imaging of the renal arteries in conjunction with a fast interrupted steady-state (FISS) readout. 3D partition thickness ranged from 0.65mm to 0.86mm with in-plane resolution of 0.5mm (after interpolation). For optimized versions of tsSOS-QISS, the separate in-plane and venous saturation RF pulses used for 2D QISS were supplanted by a single frequency offset corrected inversion (FOCI) RF pulse that extended to the top of the imaging slab and inferiorly below the slab to suppress venous signal. Optimization strategies further involved tailoring the RF excitation profile, optimizing the slice oversampling factor and slab overlap, and applying partial Fourier along the slice direction.Results
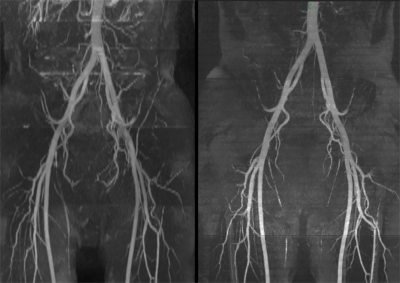
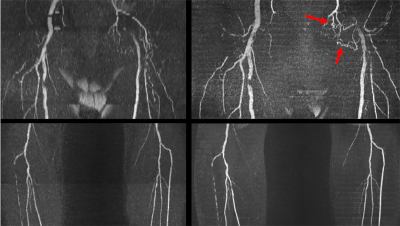
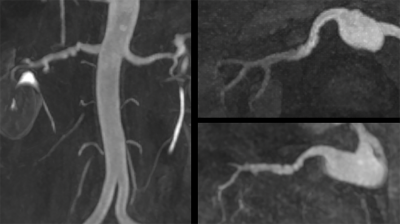
Optimal imaging parameters for tsSOS-QISS of the peripheral arteries were determined to include: 18 partitions per slab, slab thickness = 11.7mm for 0.65mm partition thickness vs. 15.5mm for 0.86mm thickness, RF pulse duration = 1400us with time-bandwidth product = 6.4, slice oversampling = 33.3%, slice partial Fourier factor = 6/8, slab overlap = 28%. Using these imaging parameters, both VBA and flow-related saturation artifact were negligible. Overall scan time was approximately twice as long for tsSOS-QISS using 0.86mm-thick 3D partitions (1.72mm acquired) compared with 2D QISS using 3.0mm-thick slices for equivalent scan regions. tsSOS-QISS consistently improved image quality and signal-to-noise ratio compared with 2D QISS. Vessel wall sharpness and branch detail were improved due to reduced partial volume averaging (Figure 1). Flow-related saturation artifact was minimal or absent, even in patients with severe multifocal disease (Figure 2). Blurring in multiplanar reformats was greatly reduced with the tsSOS-QISS approach due to a combination of near-isotropic spatial resolution and rectangular profiles for the 3D partitions. For renal artery MRA, single breath-hold tsSOS-QISS FISS image quality was comparable to much lengthier free-breathing acquisitions (Figure 3).Discussion
After optimization, the tsSOS-QISS MRA technique provides a scan efficiency that approaches that of the existing 2D QISS technique, but with greatly improved image quality. Unlike 2D QISS, tsSOS-QISS allows near-isotropic spatial resolution with rectangular slice profiles that greatly improve the quality of multiplanar reformats and more closely approximate the spatial resolution afforded by peripheral CTA.Conclusion
Using the tsSOS-QISS technique, a whole leg non-contrast 3D MRA can be obtained in less than 20 minutes and can be supplemented by breath-hold evaluation of the renal arteries. Image quality is superior to 2D QISS, while the capability for near-isotropic high spatial resolution, avoidance of contrast administration and ionizing radiation, and absence of artifacts from calcific plaque may make it an attractive alternative to CTA, especially in patients at risk for toxicity from iodinated contrast or with high prevalence of vascular calcifications. Further clinical evaluation appears warranted to determine the diagnostic accuracy and relative utility compared with CTA.Acknowledgements
FUNDING SOURCES: NIH grants R01 HL137920 and R01 HL130093 We wish to acknowledge Nondas Leloudas for assisting with scanning and data collection.References
1. J Magn Reson Imaging. 2019; 49(2):355-373.
2. Clin Radiol. 2019; 74(1):29-36.
3. Magnetic Resonance in Medicine. 2019. doi: 10.1002/mrm.28032.
Figures