2153
Whole-body MRI to assess subclinical cardiovascular disease and frailty development1Radiology, Johns Hopkins University, Baltimore, MD, United States, 2Johns Hopkins University, Baltimore, MD, United States, 3Canon Medical Research USA, Mayfield Village, OH, United States, 4Canon Medical Systems, Kanagawa, Japan
Synopsis
Whole body non-contrast magnetic resonance angiography and Dixon imaging hold potential for monitoring and quantitative assessment of global plaque burden and cardiometabolic disease in frailty.
INTRODUCTION
Currently millions of elderly individuals are considered frail, and their number is projected to rise steadily in the coming years.2,3 This, together with the burdensome clinical correlates of frailty, impose increasing demands on healthcare systems worldwide. The overall research objectives were: (1) Identify specific imaging phenotypes of fibrofatty infiltration and subclinical atherosclerosis that differentiate frail from non-frail individuals as assessed by the physical frailty phenotype, (2) Demonstrate the utility of advanced imaging markers in clinical frailty research and understanding underlying aging pathways.METHODS
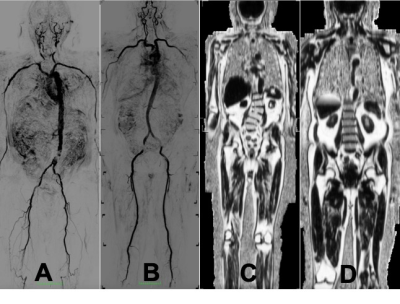
Community dwelling older adults were recruited from an aging studies registry. Screening criteria based on the five measures included in the Frailty Phenotype were used.1 The screen ascertains subjective and objective weakness, slow gait speed, decreased mobility, and weight loss. To be declared frail by these criteria, subjects had 3, 4, or 5 components of this exam. To be declared non-frail, subjects must have 0 positive frailty factors. The phenotype consists of the following measures: (1) Grip Strength measured by a dynamometer; (2) Walking Speed timed over 15 feet at their usual pace; (3) Weight loss of more than 5% of their body weight in the previous year; (4) Exhaustion assessed using two items from the CES-D Depression Scale - A) I felt that everything I do is an effort. B) I cannot get going; (5) Physical Activity assessed using the modified Minnesota Leisure Time Activities scale, which involves 18 questions on the amount of activities performed in a week. MRI was performed using a Canon Galan 3T with dedicated coils. The entire scan was performed within 50 minutes for each of the participants and involved no contrast administration. Participants were positioned supine using the following MR coils: 16 Channel Atlas Neurovascular Head, Atlas Spine, 2 Atlas body, and the 16 Channel Flex Coil. The participant was positioned on the center of the table with both arms located by their sides and legs internally rotated. Dual-echo 3D Dixon techniques were employed to assess percent fat quantification with imaging parameters – TR/TE = 5.1/(1.1, 2.8) ms, flip angle = 12 deg, slice thickness = 3-5 mm, FOV = 42x42 cm, Matrix 256x256. Users defined regions-of interest for each of seventy-eight different muscles comprising five different muscle groups (regions) –pectoral, forearm (or upper limb), pelvic, thigh, and calf muscles. In addition, fat in the liver was also quantified. ECG-prepared Fresh Blood Imaging (FBI) for non-contrast MR angiography was used for assessment of atherosclerotic burden.4 Flow-Spoiled 3D ultrafast spin echo in half Fourier acquisitions were used for fast acquisitions, with the phase encoding direction, parallel to vessel direction. Users determined significant plaques semi-automatically for 22 (including left and right) different arterial territories – internal and common carotid arteries, vertebral, subclavian, thoracic aorta, abdominal aorta, iliac, femoral, popliteal, anterior and posterior tibial, and peroneal arteries. Plaques were scored according to 4 categories – normal, <70% stenosis, 70-99% stenosis, and completely occluded. A composite atheroma burden score based on all vessels was calculated as (Sum of vessel scores)/(number of vessels).5 Modified look-locker imaging was used to assess native T1 times of the myocardium, liver and skeletal muscle, and used as surrogate markers of diffuse interstitial fibrosis: TR/TE = 2.6/1 ms, 1.5 x1.5 mm2 in-plane resolution, 10 mm slice thickness .6 Aortic length (length perpendicular to aortic cross-section from the aortic root to the aorto-iliac bifurcation) indexed to height and aortic tortuosity (aortic length/length of straight line from start to end of aorta) will be calculated as markers of aortic elongation.7RESULTS
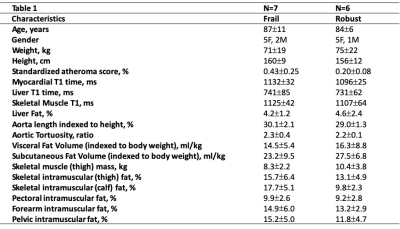
Preliminary analysis from 7 frail individuals and 6 age-matched robust controls is presented. Of 7 frail individuals, one had a prior heart attack; one was previously diagnosed with heart failure. The atheroma score (p<0.05), aortic length, and aortic toruosity were all higher in frail as compared to robust individuals indicative of higher atherosclerotic burden and vascular stiffness. Subcutaneous and visceral adipose tissue volumes were lower in frail as compared to robust individuals. However, myocardial, liver and skeletal muscle T1 times were higher indicative of greater diffuse interstitial fibrosis (p<0.05 for myocardium). Intramuscular fat was measured across five different regions – pelvis, forearm, pectus, thigh, and calf; the average intramuscular fat percent was higher in frail compared to robust individuals indicative of higher fatty infiltration (p<0.05 for calf). The total thigh muscle volume was lower in frail as compared to robust controls (p<0.05).DISCUSSION and CONCLUSION
In this pilot study, we have demonstrated the possibility of using comprehensive and non-contrast whole-body MR angiography to quantify atherosclerosis burden as well as using Dixon imaging of muscle tissue to assess underlying cardiometabolic disease. We also used native T1 estimates to assess diffuse interstitial fibrosis. Frailty onset is characterized by reduced energy reserves, lack of resilience, sarcopenia, lack of muscle volume and fatigability. Measures of fibrofatty infiltration and nonsignificant atherosclerotic plaque burden may have the potential to identify underlying subclinical cardiometabolic disease. These measures may be useful to monitor and quantify progression towards frailty. In addition, the lack of contrast administration, allows the participation of those with renal dysfunction as well.Acknowledgements
This work was partially supported by the Johns Hopkins University Pepper Center Older American Independence Center ( Funds to support this OAIC study were provided by the Johns Hopkins University Older Americans Independence Center of the National Institute on Aging (NIA) under award number P30AG021334) and with support from Canon Medical Research USA.References
1. Rodriguez-Mañas, L. & Fried, L. P. Frailty in the clinical scenario. Lancet 385, e7–e9 (2015).
2. Walston, J. Frailty in older adults. Oxford Textbook of Geriatric Medicine (2017).
3. Fried, L. P. et al. Frailty in older adults: evidence for a phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 56, (2001).
4. Miyazaki, M. & Akahane, M. Non‐contrast enhanced MR angiography: Established techniques. J Magn Reson Imaging 35, 1–19 (2012).
5. Weir-McCall, J. R. et al. Whole-body cardiovascular MRI for the comparison of atherosclerotic burden and cardiac remodelling in healthy South Asian and European adults. Br J Radiology 89, 20160342 (2016).
6. Messroghli, D. R. et al. Modified Look‐Locker inversion recovery (MOLLI) for high‐resolution T1 mapping of the heart. Magnet Reson Med 52, 141–146 (2004).
7. Franken, R. et al. Increased aortic tortuosity indicates a more severe aortic phenotype in adults with Marfan syndrome. Int J Cardiol 194, 7–12 (2015).
Figures