2150
Semi-automatic Arterial Territorial Segmentation using ASL-based Dynamic 4D MRA without Vessel-Encoded Labeling1USC Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 2Department of Neurology, University of Southern California, Los Angeles, CA, United States, 3Razor Labs, Tel Aviv, Israel, 4Medicine & Sagol School of Neuroscience, Tel Aviv University, Tel Aviv, Israel, 5Peking Union Medical College Hospital, Beijing, China
Synopsis
Characterizing vascular territorial structures and hemodynamics from a single artery can provide crucial information for the assessment and treatment of cerebrovascular disorders such as arteriovenous malformations, Moyamoya disease, and aneurysms. In a different approach compared to vessel-selective MR angiography (MRA), here we presented a semi-automatic post-processing technique to segment vascular territories using pulsed arterial spin labeling 4D MRA. Our results demonstrated the feasibility of using 4D MRA in conjunction with arterial territorial segmentation to visualize vascular territories and quantify blood flow supply from individual arteries.
Introduction
The ability to characterize vascular structure and hemodynamics from a single artery is of importance in clinical diagnosis and treatment of cerebrovascular disorders; for example, in selection of a donor artery for the bypass surgery. Digital subtraction angiography is the main imaging modality that allows for vascular imaging from a single vessel. Recent vessel-encoded (VE) arterial spin labeling (ASL)-based MR angiography (MRA) can offer vessel-selective imaging. However, multiple-encoding steps are necessary to differentiate feeding arteries and prior knowledge of the position of the feeding arteries is always required, leading to lengthened scan time and extra effort/time for scan preparation. Pulsed-ASL-based time-resolved four-dimensional MRA (4D MRA) provides dynamic information of the flow through the cerebral vasculature with high spatial and temporal resolution1,2 and its clinical utility in depicting dynamic flow patterns have been demonstrated in Moyamoya disease3, aneurysm4, and arteriovenous malformation5. In this study, we presented a novel semi-automatic post-processing algorithm to equip 4D MRA with additional features including vessel territorial mapping and regional volumetric blood flow (rVBF) quantification from a single vessel, which could provide complementary information for clinical studies at no expense of extra scan time.Methods
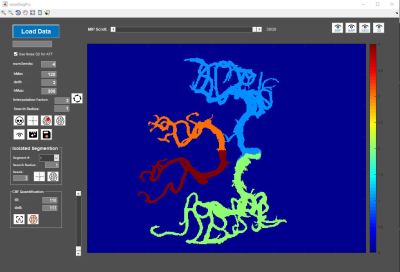
Participants and Imaging protocol: PASL-based 4D MRA sequence was performed using a Siemens Prisma 3T Scanner on four healthy subjects and one Moyamoya patient after superficial temporal artery (STA)-middle cerebral artery (MCA) bypass surgery. Imaging parameters included voxel size=1×1×1.5mm3, temporal resolution=25ms, and number of phases=30. A 2D PC-MRI sequence (resolution=0.5×0.5×5.0 mm3, VENC=80 or 70 cm/s, TE/TR=6.29/21.75 ms, flip angle=15°) was performed to measure mean flow rates of four main branches of the circle of Willis including left and right middle cerebral arteries (LMCA and RMCA), left and right posterior cerebral arteries (LPCA and RPCA).Development of semi-automatic algorithm pipeline for vessel territorial segmentation in 4D MRA: We developed a graphical user interface-based software (VSegPro) using MATLAB to perform 4D MRA preprocessing and vessel territorial segmentation (Figure 1). 4D MRA images were obtained by subtracting label images from control images. Following image denoising using an optimized nonlocal means filter6, the residual skull signal in 4D MRA images was removed using a freehand marquee tool and the desired number of seed points were marked manually on the temporal maximum intensity projection (MIP) image in the axial view.
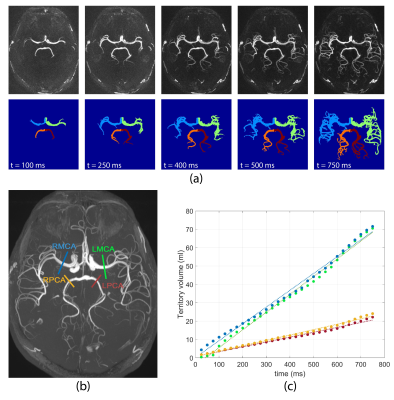
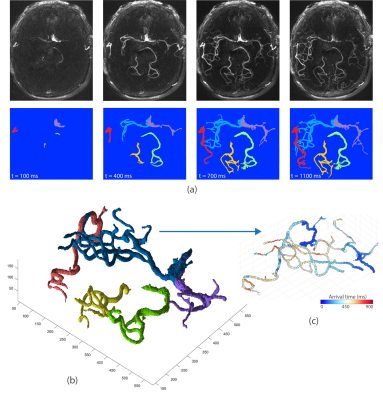
Territorial segmentation was carried out on 4D MRA images using a bolus arrival time (BAT) based region growing algorithm7. In brief, first, BAT in each voxel was estimated as the first time point at which the 4D MRA signal was larger than 3×standerd deviation of the background noise8. Next, the selected seed points were used to initiate the region growing for each territory based on variable 4D MRA signal threshold which was progressively decreased so that vessels with larger spatial separation (proximal vessels) were segmented first. At each time point, only the voxels with BAT smaller than the current time were retained. In order to avoid overlapping territories, during the region growing process, each territory was considered as a blocking zone for other territories. rVBF for each territory was quantified as the slope of the linear regression between territory volume and time (Figure 2-c).
Upon completion of the automated segmentation, dynamic MRA in each territory can be visualized using time series of 2D maximum intensity projection (MIP) images or a 3D rendered volume.
Results and Discussion
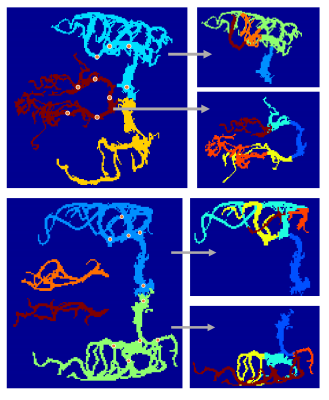
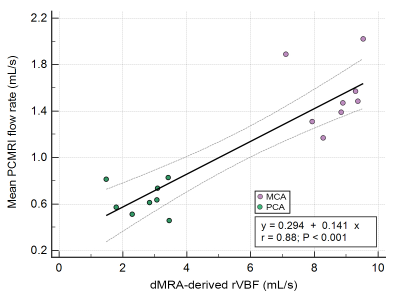
4D MRA data acquisition and territorial segmentation were performed successfully in all subjects. Figure 2 shows an example of time series of MIP images in the axial view as well as corresponding cerebrovascular territorial segmentations from each main branches. Each territory can further be segmented into desired number of sub-territories, depending on the locations of seed points. As shown in Figure 3, sub-territories from M2/3 and P2/3 can be well appreciated. Regression analysis between 4D MRA and PC-MRI measurements of various territories showed a significant correlation between 4D MRA-derived rVBF values and mean PC-MRI flow rates (r=0.88, p<0.001, Figure 4), indicating that 4D MRA is capable of quantifying relative blood flow through a vessel segment. It should be noted that rVBF values were impacted by the partial volume effects and the threshold values used during region growing and as such, were systematically larger than PC-MRI-measured mean flow rate.The ability to distinguish the main and collateral routes of blood supply to affected brain regions in cerebrovascular disease is crucial. Figure 5 shows 4D MRA from a Moyamoya case after SAT-MCA bypass surgery. The blood supply from donor artery (red) can be distinguished from the blood flow from the recipient right MCA (blue). Cerebrovascular territorial imaging using 4D MRA may help to improve the preoperative assessments and postoperative monitoring of Moyamoya patients who undergo direct bypass surgery.
Conclusion
We proposed a semi-automatic post-processing algorithm by using bolus arrival time (BAT) based region-growing to obtain vascular territories in 4D MRA. Compared to VE-ASL MRA, this method provides more freedom to acquire vascular territories at different levels of vessel segments (such as M1/2/3 and P1/2/3). 4D MRA with arterial territory mapping may provide additional useful information for diagnosis and therapeutic decision making in different neurovascular pathologies.Acknowledgements
This work is supported by grants of NIH K25-AG056594 and AHA 16SDG29630013.References
1. Yan, L., et al. Unenhanced dynamic MR angiography: high spatial and temporal resolution by using true FISP–based spin tagging with alternating radiofrequency. Radiology 256, 270-279 (2010).
2. Bi, X., Weale, P., Schmitt, P., Zuehlsdorff, S. & Jerecic, R. Non‐contrast‐enhanced four‐dimensional (4D) intracranial MR angiography: a feasibility study. Magnetic resonance in medicine 63, 835-841 (2010).
3. Uchino, H., et al. A novel application of four-dimensional magnetic resonance angiography using an arterial spin labeling technique for noninvasive diagnosis of Moyamoya disease. Clinical neurology and neurosurgery 137, 105-111 (2015).
4. Wu, H., Block, W.F., Turski, P.A., Mistretta, C.A. & Johnson, K.M. Noncontrast‐enhanced three‐dimensional (3D) intracranial MR angiography using pseudocontinuous arterial spin labeling and accelerated 3D radial acquisition. Magnetic resonance in medicine 69, 708-715 (2013).
5. Iryo, Y., et al. Evaluation of intracranial arteriovenous malformations with four-dimensional arterial-spin labeling–based 3-T magnetic resonance angiography. Journal of computer assisted tomography 40, 290-296 (2016).
6. Coupé, P., et al. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE transactions on medical imaging 27, 425-441 (2008).
7. Geri, O., et al. Vascular territorial segmentation and volumetric blood flow measurement using dynamic contrast enhanced magnetic resonance angiography of the brain. Journal of Cerebral Blood Flow & Metabolism 37, 3446-3456 (2017).
8. Shao, X., et al. Quantification of intracranial arterial blood flow using noncontrast enhanced 4D dynamic MR angiography. Magnetic resonance in medicine 82, 449-459 (2019).
Figures