2141
Similarity-Based Angiography (SIMBA) for Fast Reconstruction of Static Whole-Heart Coronary MR Images from Free-Running Acquisitions1Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 2Advanced Clinical Imaging Technology, Siemens Healthcare, Lausanne, Switzerland, 3Children’s Hospital of Philadelphia, Philadelphia, PA, United States, 4Center for Biomedical Imaging (CIBM), Lausanne, Switzerland
Synopsis
SIMilarity-Based Angiography (SIMBA) is a data-driven method for fast reconstruction of static whole-heart coronary MR angiograms from free-running acquisitions. By assessing the similarity of periodically acquired k-space readouts in the superior-inferior direction, motion-consistent angiograms can be obtained without making stringent assumptions about physiological motion. SIMBA demonstrated potential to reconstruct cardiac and respiratory motion-consistent images with visible coronary arteries both from non-contrast volunteer acquisitions at different field strengths and contrast-enhanced scans of pediatric patients. SIMBA provided improved sharpness and contrast compared to images from all the acquired data and similar vessel conspicuity as end-expiratory mid-diastolic frames of motion-resolved compressed sensing reconstructions.
Introduction
Whole-heart coronary MRA is commonly performed using ECG-triggering and respiratory gating which are often associated with complicated acquisition planning and unpredictable scan times.1 Recently introduced free-running sequences2–3 reduce planning complexity, guarantee fixed scan times, and offer the possibility to obtain dynamic information by retrospectively sorting the continuously collected data into different motion states.2–6 Nevertheless, these approaches require a priori assumptions regarding physiological frequencies, which may vary during scans or among patients, and often exploit time-consuming motion-resolved compressed sensing reconstruction algorithms4. This work aims at combining operator-friendly free-running acquisitions with a novel fast image reconstruction method. We hypothesize that a subject-specific motion-consistent subset of k-space data can be identified without stringent physiological assumptions, and that standard reconstruction thereof provides a motion-suppressed static whole-heart image with conspicuous coronary arteries.Methods
We propose a SIMilarity-Based Angiography (SIMBA) reconstruction technique for free-running 3D radial whole-heart coronary MRA scans. In brief, k-means7 is used to cluster the MR data based on the similarity of regularly acquired superior-inferior readouts (Fig.1-SIMBA). Subsequently, standard gridding reconstruction of the most populated cluster produces a static 3D image.Validation was performed using variants of a 3D free-running sequence3 with a prototype radial Phyllotaxis trajectory8. N=15 free-running datasets were acquired with three different protocols: ferumoxytol9 contrast-enhanced gradient echo (CE-GRE) under respiratory ventilation (5 pediatric patients), non-contrast fast-interrupted steady state (FISS)6 (5 healthy volunteers), and fat-saturated bSSFP (FS-bSSFP)3 (5 healthy volunteers). Data were acquired on 1.5T MAGNETOM Avantofit (5xCE-GRE) and Aera (3xFISS, 5xFS-bSSFP) and 3T MAGNETOM Prismafit (2xFISS) clinical scanners (Siemens Healthcare, Erlangen, Germany). Main common parameters: Resolution (1.1 mm)3, FOV (220 mm)3, 5181-5749 radial interleaves of 22-24 readouts. The fixed acquisition times were: CE-GRE 7:03min, FISS 7:53-9:33min, FS-bSSFP 14:17min. The ECG was recorded as reference.
For comparison, three whole-heart reconstructions were obtained per dataset: by gridding all collected data (All Data), from SIMBA, and by selecting an end-expiratory mid-diastolic image from a fully self-gated cardiac and respiratory motion-resolved (4 respiratory bins, 50ms cardiac resolution) compressed sensing reconstruction from a free-running framework (FRF)5. For SIMBA, the percentage of automatically selected data was computed, and the reconstruction time recorded. Moreover, the data's physiological origin was evaluated using ECG-timestamps and the FRF's respiratory self-gating signal. For all reconstructions, sharpness was measured by fitting sigmoid functions to the blood-myocardium interface.10 Blood-myocardium contrast ratio was quantified by comparing average signal intensities in regions of interest. Furthermore, the visibility of the coronary ostia was assessed by one author (2.5yrs.’experience). For SIMBA and FRF, the visible length and sharpness of the right coronary artery (RCA) and the combined left main (LM) + left anterior descending (LAD) coronary arteries were quantified using Soap-Bubble.11 For statistical comparisons, two-sided paired sample t-tests, after evaluating normality (Jarque-Bera12), and McNemar's tests (ostia) were used with p<0.05 after Bonferroni correction being considered statistically significant.
Results
On average, the cluster selected by SIMBA contained 12.8%±1.7% of the acquired data, corresponding to 27.8%±3.7% of the radial Nyquist limit. The image reconstruction time for SIMBA was less than 30s on a desktop computer, while reconstructing a motion-resolved FRF-dataset takes several hours.Visually, SIMBA removed most of the motion blurriness seen in the All Data-reconstructions and provided comparable quality to FRF, albeit with slightly increased noise (Fig.2). The selected SIMBA-cluster contained mainly end-expiratory data (78%±26% originated from the two most end-expiratory FRF bins). Conversely, three distinct scenarios were observed in the cardiac dimension (Fig.3).
Both SIMBA and FRF provided significantly higher blood-myocardium sharpness than All Data, but no significant difference was observed between the two (Fig.4a). Though blood-myocardium contrast differed between the three protocols (Fig.4b), normalized values only showed a significant improvement between SIMBA and All Data (Fig.4c). Significantly more ostia were visible with SIMBA and FRF compared to All Data (All Data: 6/30, SIMBA: 25/30, FRF: 29/30) but only non-significant differences were seen between SIMBA and FRF concerning ostia, vessel length and sharpness (Fig.5).
Discussion
SIMBA mainly selected end-expiratory data from well-defined phases of the cardiac cycle, which together with the fast-moving coronary arteries being generally visible, points towards motion-consistent data in the selected cluster. The findings that SIMBA-images showed better blood-myocardium sharpness and contrast than All Data-images and similar coronary and blood-myocardium sharpness as FRF-images support our hypothesis that a sharp whole-heart coronary MRA can be obtained without making a priori physiological assumptions. The non-significant differences between SIMBA and FRF might be explained by the fact that one SIMBA-image, reconstructed without compressed sensing, directly uses more data than one FRF-frame (~3% Nyquist), but FRF’s regularization means that information is shared between frames. Potentially, non-trivial ways of combining data from non-adjacent phases in the cardiac cycle can be identified with SIMBA, as seen when early systolic and mid-diastolic data clustered together (Fig.3). Intuitively, this result is explainable by the fact that the heart has a similar anatomical shape during those two phases. Finally, SIMBA may complement motion-resolved compressed sensing reconstructions by making images available quickly at the scanner console.Conclusion
SIMBA provides a means of automatically selecting motion-consistent data from free-running acquisitions of the heart without making a priori physiological assumptions, allowing for fast reconstruction of whole-heart coronary MRA with comparable quality to that obtained with a much longer iterative reconstruction.Acknowledgements
No acknowledgement found.References
1. Dweck MR, Puntman V, Vesey AT, Fayad ZA, Nagel E. MR Imaging of Coronary Arteries and Plaques. JACC Cardiovasc. Imaging 2016;9:306–316 doi: 10.1016/j.jcmg.2015.12.003.
2. Pang J, Sharif B, Fan Z, et al. ECG and navigator-free four-dimensional whole-heart coronary MRA for simultaneous visualization of cardiac anatomy and function. Magn. Reson. Med. 2014;72:1208–1217 doi: 10.1002/mrm.25450.
3. Coppo S, Piccini D, Bonanno G, et al. Free-running 4D whole-heart self-navigated golden angle MRI: Initial results. Magn. Reson. Med. 2015;74:1306–1316 doi: 10.1002/mrm.25523.
4. Feng L, Coppo S, Piccini D, et al. 5D whole-heart sparse MRI. Magn. Reson. Med. 2018;79:826–838 doi: 10.1002/mrm.26745.
5. Di Sopra L, Piccini D, Coppo S, Stuber M, Yerly J. An automated approach to fully self‐gated free‐running cardiac and respiratory motion‐resolved 5D whole‐heart MRI. Magn. Reson. Med. 2019;82:2118–2132 doi: 10.1002/mrm.27898.
6. Bastiaansen JAM, Piccini D, Di Sopra L, et al. Natively fat‐suppressed 5D whole‐heart MRI with a radial free‐running fast‐interrupted steady‐state (FISS) sequence at 1.5T and 3T. Magn. Reson. Med. 2020;83:45–55 doi: 10.1002/mrm.27942.
7. MacQueen J. Some methods for classification and analysis of multivariate observations. In: Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Volume 1: Statistics. Fifth Berkeley Symposium on Mathematical Statistics and Probability. Berkeley, Calif.: University of California Press; 1967. pp. 281–297.
8. Piccini D, Littmann A, Nielles-Vallespin S, Zenge MO. Spiral phyllotaxis: The natural way to construct a 3D radial trajectory in MRI. Magn. Reson. Med. 2011;66:1049–1056 doi: 10.1002/mrm.22898.
9. Prince MR, Zhang HL, Chabra SG, Jacobs P, Wang Y. A pilot investigation of new superparamagnetic iron oxide (ferumoxytol) as a contrast agent for cardiovascular MRI. J. Xray. Sci. Technol. 2003;11:231–40.
10. Ahmad R, Ding Y, Simonetti OP. Edge Sharpness Assessment by Parametric Modeling: Application to Magnetic Resonance Imaging. Concepts Magn. Reson. Part A. Bridg. Educ. Res. 2015;44:138–149 doi: 10.1002/cmr.a.21339.
11. Etienne A, Botnar RM, Van Muiswinkel AMC, Boesiger P, Manning WJ, Stuber M. “Soap-Bubble” visualization and quantitative analysis of 3D coronary magnetic resonance angiograms. Magn. Reson. Med. 2002;48:658–666 doi: 10.1002/mrm.10253.
12. Jarque CM, Bera AK. A Test for Normality of Observations and Regression Residuals. Int. Stat. Rev. / Rev. Int. Stat. 1987;55:163–172 doi: 10.2307/1403192.
Figures
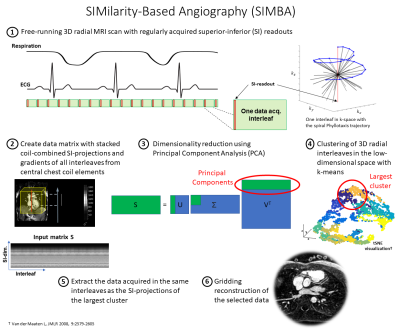
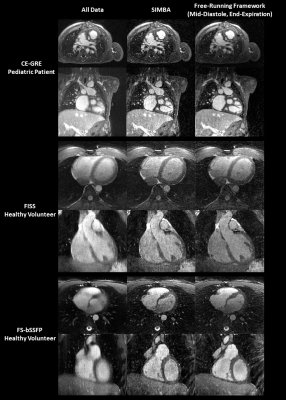
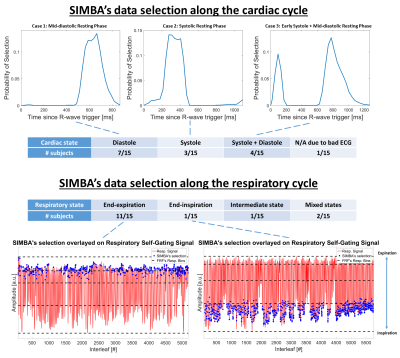
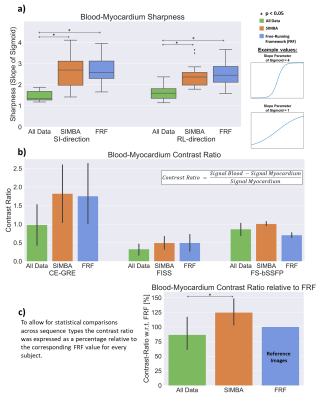
Figure 4.
a) The blood-myocardium sharpness in the superior-inferior (SI) and right-left (RL) directions obtained from fitting sigmoid functions to the interface and using the average of three slope parameter values per direction as a sharpness measure.
b) Barplots showing the average blood-myocardium contrast ratio with confidence intervals. As expected, CE-GRE provided best contrast.
c) To allow for statistical comparisons with N=15, normalization was performed by expressing the individual subjects’ contrast ratio values as a percentage of the corresponding FRF values.
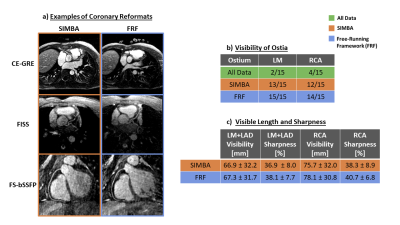
Figure 5.
a) Examples of coronary reformats from SIMBA and FRF. From this CE-GRE scan of a pediatric patient with an anomalous RCA, SIMBA extracted a systolic image which explains the anatomical difference compared to the mid-diastolic FRF image.
b) In images from All Data, only a handful ostia were considered visible whereas in the SIMBA and FRF images the majority were seen.
c) No significant differences in visible vessel length and sharpness of the LM + LAD and RCA were found in the vessels were both SIMBA and FRF could visualize the ostia, although FRF provided slightly higher values.