2139
Imaging of the Pulmonary Vasculature in Congenital Heart Disease Using Relaxation-Enhanced Angiography Without Contrast and Triggering
Lenhard Pennig1, Anton Wagner1, Kilian Weiss2, Simon Lennartz1, Jan-Peter Grunz3, David Maintz1, Kai Roman Laukamp1, Tilman Hickethier1, Claas Philip Naehle1, Alexander Bunck1, and Jonas Doerner1
1Institute for Diagnostic and Interventional Radiology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany, Cologne, Germany, 2Philips GmbH, Hamburg, Germany, 3Department of Diagnostic and Interventional Radiology,, University Hospital Würzburg, Würzburg, Germany, Würzburg, Germany
1Institute for Diagnostic and Interventional Radiology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany, Cologne, Germany, 2Philips GmbH, Hamburg, Germany, 3Department of Diagnostic and Interventional Radiology,, University Hospital Würzburg, Würzburg, Germany, Würzburg, Germany
Synopsis
Patients with Congenital heart disease (CHD) require repetitive imaging of the pulmonary vasculature with CE-MRA showing risks like anaphylactic reactions and uncertain long-term effects of gadolinium deposition in the brain. We adapted a Compressed SENSE (CS) accelerated 3D REACT (navigator- and ECG-triggered) to the pulmonary vessels and compared it to standard 4D CE-MRA in the clinical routine. With REACT providing significant higher image quality and slightly higher interobserver agreement in a reasonable scan time, it may be regarded as a clinically applicable alternative for patients in need of repetitive imaging of the pulmonary vasculature without the use of contrast agents.
Introduction
Congenital heart disease (CHD) comprises a wide range of different manifestations regarding the cardiovascular system with cardiac MRI being established as the non-invasive imaging technique of choice to evaluate the different vascular territories of the thorax1. In this context, contrast-enhanced MR-angiography (CE-MRA) has proven to sufficiently detect vascular abnormalities and has shown technical progress over the past decades with the development of time resolved 4D CE-MRA 2. However, use of gadolinium contrast shows several risks and limitations such as nephrogenic systemic fibrosis in end-stage renal disease, anaphylactic reactions and uncertain long-term effects of gadolinium deposition in the brain 3-5.Hence, many non-CE-MRA techniques have been developed in the past, with steady-state free precession (SSFP) and balanced SSFP (bSSFP) being the most widely used for the assessment of the pulmonary vasculature in patients with CHD 6-8. Recently, a novel 3D Relaxation-Enhanced Angiography without Contrast and Triggering (REACT) sequence was introduced comprising of non-volume-selective short tau inversion recovery (STIR) and T2 preparation pulses with a dual gradient echo Dixon (mDIXON XD) 3D readout. It combines benefits of SSFP with robust suppression of background and fat for flow-independent non-CE-MRA 9.
The purpose of this study was to investigate the feasibility of the novel REACT sequence for imaging of the pulmonary arteries and veins without the use of contrast agent in patients with CHD and to compare measurement values and image quality to standard 4D CE-MRA.
Methods
Retrospective, IRB-approved single-center study including 25 consecutive patients (mean age 39±20 years, 15 males) with known or suspected CHD receiving a standard protocol in the clinical routine including both, flow-independent 3D isotropic REACT (using Compressed SENSE factor 9 for acceleration of image acquisition) and 4D CE-MRA on a whole body 1.5 T MRI system (Philips Ingenia, Philips Healthcare, Best, the Netherlands) (June 2018 – April 2019). Given the sufficient background suppression of mDIXON XD in combination with T2 preparation for cardiovascular applications, no STIR preparation was applied for REACT with ECG-triggering (end-diastolic) and respiratory navigator-triggering being added to compensate for cardiac and respiratory motion. For 4D CE-MRA, no triggering was applied.Two fully blinded radiologists independently executed measurements in manual perpendicular alignment on source images of REACT and 4D CE-MRA on seven dedicated points (inner edge): Main pulmonary artery (MPA), right and left pulmonary artery, right superior and inferior pulmonary vein, left superior (LSPV) and inferior pulmonary vein (LIPV). Image quality for arteries and veins was evaluated on a four-point scale in consensus (1 non-diagnostic – 4 excellent image quality). Measurement points were excluded when they could not be assessed due to severe pulsation artifacts.
Results
23 of 25 patients presented a CHD with 10 patients receiving surgery for CHD prior to the examination. REACT showed an average total acquisition time of 07:01±02:44 min (depending on the patient´s breathing frequency and heart rate) depicting the whole thorax. Measurements in 4D CE-MRA reached higher diameter values compared to REACT, at the pulmonary arteries with significant difference (e. g. MPA: mean difference of 0.408 cm, p=0.002). There was a high interobserver agreement for both methods at the pulmonary arteries (ICC ≥ 0.96). At the pulmonary veins, REACT showed a slightly higher agreement, pronounced at LSPV (ICC 0.946 vs. 0.895). REACT showed significant better image quality at the pulmonary arteries (3.84 vs. 3.32, p=0.0002) and veins (3.32 vs. 2.72, p=0.0152) than 4D CE-MRA. Using 4D CE-MRA, measurement was not possible at the LSPV in one patient and at the LIPV in three patients due to impaired image quality. In REACT, all measurements were conducted sufficiently.Discussion
The results of this study indicate that navigator- and ECG-triggered 3D REACT is highly suitable for imaging of the pulmonary vasculature in patients with CHD providing higher image quality and slightly higher interobserver agreement than 4D CE-MRA.Alongside other studies comparing non-ECG-triggered CE-MRA with ECG-triggered non-CE-MRA (SSFP, end-diastolic) for the imaging of the pulmonary vasculature, 4D CE-MRA showed higher measurement values of the pulmonary vessels compared to REACT, mainly due to pulsation and breathing artifacts in 4D CE-MRA 7,10-13. Being routinely applied to imaging of the thoracoabdominal vessels, bSSFP shows drawbacks such as sensitivity to off-resonance effects and insufficient fat suppression caused by B0 heterogeneities in the magnetic field and disruptions of the steady state due to highly pulsatile flow or motion 6,14,15. These effects are pronounced in higher magnetic fields and large field of views (FOVs). When applied in large FOVs, long acquisition time is required consequently limiting its use in the clinical routine. Contrary, the mDIXON readout of REACT provides reduced sensitivity to inhomogeneities in the magnetic field and robust fat suppression, even in large FOVs and at higher magnetic fields. Given the Compressed SENSE acceleration, REACT showed a lower scan time than 3D SSFP and bSSFP for the same kind of investigation and FOV 6.
Conclusions
Navigator- and ECG-triggered 3D REACT allows for robust imaging of the pulmonary vasculature in CHD with higher image quality and slightly higher interobserver agreement than 4D CE-MRA without the need of gadolinium contrast. Given its short acquisition time, it represents a clinically applicable alternative for patients with CHD in need of repetitive imaging of the pulmonary vessels.Acknowledgements
None.References
- Ntsinjana HN, Hughes ML, Taylor AM, et al. The role of cardiovascular magnetic resonance in pediatric congenital heart disease, J. Cardiovasc. Magn. Reson. 2011; 21;13:51
- Vogt FM, Theysohn JM, Michna D, et al. Contrast-enhanced time-resolved 4D MRA of congenital heart and vessel anomalies: Image quality and diagnostic value compared with 3D MRA, Eur. Radiol. 2013; 23(9):2392-404.
- Gulani V, Calamante F, Shellock FG, et al. Gadolinium deposition in the brain: summary of evidence and recommendations, Lancet Neurol. 2017; 16(7):564-570.
- Jung J-W, Kang H-R, Kim M-H, et al. Immediate Hypersensitivity Reaction to Gadolinium-based MR Contrast Media. Radiology. 2012; 264:414-422.
- Perazella MA. Advanced kidney disease, gadolinium and nephrogenic systemic fibrosis: The perfect storm. Curr. Opin. Nephrol. Hypertens. 2009;18:519-525.
- François CJ, Tuite D, Deshpande V, et al. Pulmonary Vein Imaging with Unenhanced Three-dimensional Balanced Steady-State Free Precession MR Angiography: Initial Clinical Evaluation, Radiology. 2009; 250(3):932-9
- Krishnam MS, Tomasian A, Malik S, et al. Three-dimensional imaging of pulmonary veins by a novel steady-state free-precession magnetic resonance angiography technique without the use of intravenous contrast agent: Initial experience. Invest. Radiol. 2009; 44(8):447-53.
- Edelman RR, Silvers RI, Thakrar, KH, et al. Nonenhanced MR angiography of the pulmonary arteries using single-shot radial quiescent-interval slice-selective (QISS): A technical feasibility study, J. Cardiovasc. Magn. Reson. 2017; 19:48.
- Yoneyama M, Zhang S, Hu HH, et al. Free-breathing non-contrast-enhanced flow-independent MR angiography using magnetization-prepared 3D non-balanced dual-echo Dixon method: A feasibility study at 3 Tesla, Magn. Reson. Imaging 2019; 63:137-146.
- Syed MA, Peters DC, Rashid H, et al. Pulmonary vein imaging: Comparison of 3D magnetic resonance angiography with 2D cine MRI for characterizing anatomy and size. J. Cardiovasc. Magn. Reson. 2005; 7(2):355-60.
- Shariat M, Schantz D, Yoo SJ, et al. Pulmonary artery pulsatility and effect on vessel diameter assessment in magnetic resonance imaging, Eur. J. Radiol. 2014; 83(2):378-83.
- Burman E.D, Keegan J, Kilner P.J., et al. Pulmonary artery diameters, cross sectional areas and area changes measured by cine cardiovascular magnetic resonance in healthy volunteers, J. Cardiovasc. Magn. Reson. 2016; 3;18:12.
- Hauser TH, Yeon SB, Kissinger KV, et al. Variation in pulmonary vein size during the cardiac cycle: Implications for non-electrocardiogram-gated imaging. Am. Heart J. 2006; 152(5):974.e1-6.
- Bangerter, NK, Çukur T, Hargreaves BA, et al. Three-dimensional fluid-suppressed T2-prep flow-independent peripheral angiography using balanced SSFP, Magn. Reson. Imaging. 2011; 29(8):1119-24
- Çukur T, Lee J.H, Bangerter NK, et al. Non-contrast-enhanced flow-independent peripheral MR angiography with balanced SSFP, Magn. Reson. Med. 2009; 61(6):1533-9.
Figures
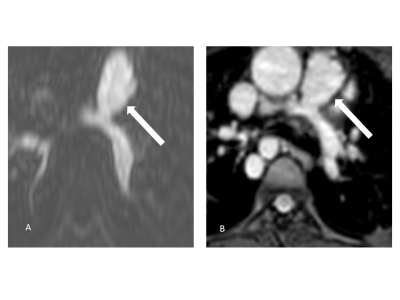
Multiplanar
reformatted source images of the main pulmonary artery (arrows) in an 11-year-old
patient with pulmonary atresia after implantation of a Contegra conduit and
multiple angioplasties of both pulmonary arteries (A: 4D CE-MRA, B: REACT). Compared to the blurred appearance in 4D CE-MRA, the
pulmonary arteries can be clearly delineated in REACT.
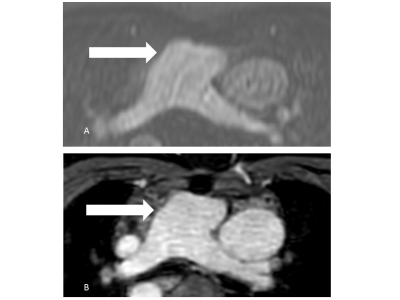
Multiplanar
reformatted source images of the main pulmonary
artery (arrows) in a 39-year-old patient
with situs inversus totalis and transposition of the great
artery (A: 4D CE-MRA, B: REACT). REACT
achieves superior delineation of the vessel wall
compared to 4D CE-MRA due to pulsation artifacts.
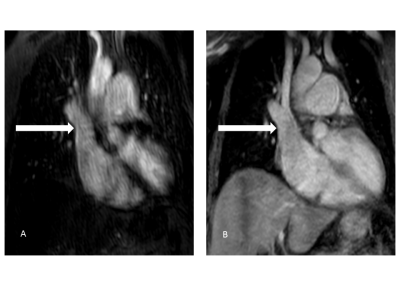
Source
images of 4D CE-MRA (A) and REACT (B) in a 56-year-old patient with sinus
venosus atrial septal defect and suspected associated anomalous pulmonary
venous return. REACT clearly shows the connection of the right superior pulmonary
vein with the superior vena cava (arrows) whereas diagnosis is hampered in 4D CE-MRA due to pulsation
artifacts.
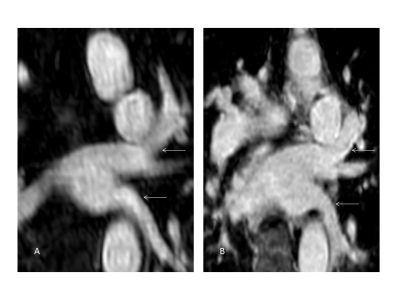
Multiplanar
reformatted source images of the left
pulmonary veins (arrows; A: 4D CE-MRA, B: REACT) in a 71-year-old patient with
patent ductus arteriosus with REACT achieving improved delineation of the vessel
wall compared to 4D CE-MRA.