2137
Non-contrast Enhanced 3D Cartesian Aortic MR Angiography in 3 minutes1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Synopsis
MR angiography is a useful technique for the diagnosis, monitoring and treatment planning of thoracic aortic disease. However, clinically used contrast-free approaches based on diaphragmatic navigator gating commonly result in long and unpredictable scan times. This study extends our previously introduced technique for non-contrast enhanced (non-CE) accelerated 3D Cartesian non-rigid motion-compensated whole-heart MR angiography for the visualization of the thoracic and suprarenal abdominal aorta from a short and efficient 3-minute scan. The proposed method was tested in five healthy subjects and two patients with cardiovascular disease, resulting in high-quality depiction of the aorta, holding promise for integration in clinical routine.
INTRODUCTION
Contrast-enhanced MR angiography (CE-MRA) with gadolinium-based contrast agents (GBCA) is a well-established technique for the assessment of the thoracic vasculature, and it is widely used for diagnosis and monitoring of a variety of aortic pathologies.1 However, concerns about the risk of side effects from GBCAs in patients with impaired renal function, and recent evidence of GBCA deposition in the brain have stimulated the development and clinical translation of alternative non-CE MRA techniques. Promising approaches based on free-breathing ECG-triggered balanced steady state free precession (bSSFP) sequences that integrated T2 preparation and diaphragmatic navigator (dNAV) gating for respiratory motion compensation were introduced over 10 years ago.2,3 Although several studies showed that the performance of both CE-MRA and non-CE bSSFP MRA techniques is comparable in terms of image quality and diagnostic value4,5, long and unpredictable scan times have limited the wide clinical adoption of dNAV non-CE aortic MRA.Recent advances in respiratory self-navigation and respiratory-resolved reconstruction for cardiac MR imaging have enabled non-CE aortic MRA with higher spatial resolution (1.5-1.7mm isotropic) from scans with predictable time of approximately 6 minutes.6,7 However, the use of non-Cartesian sampling trajectories and respiratory-resolved imaging resulted in long reconstruction times, which could hinder rapid clinical translation of these approaches. In this study, we further reduce the acquisition time of non-CE aortic Cartesian MRA imaging to enable acquisitions with high isotropic spatial resolution in a short and predictable scan of ~3min. This is achieved by extending our previously introduced accelerated non-rigid motion compensated whole-heart MR angiography approach8 for the visualization of the thoracic and suprarenal abdominal aorta.
METHODS
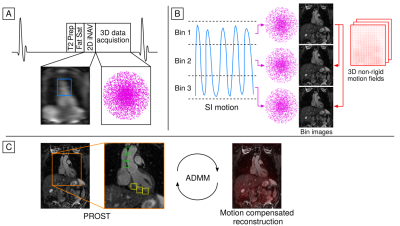
Aortic MRA data are acquired with an ECG-triggered 3D bSSFP sequence with an undersampled variable-density golden-step Cartesian trajectory with spiral profile order.9,10 Low-resolution 2D image navigators (iNAVs)11 are acquired at each cardiac cycle by spatially encoding the start-up echoes preceding the 3D MRA acquisition. Fat saturation and adiabatic T2 preparation pulses are performed to improve contrast between blood and surrounding tissue without the use of exogenous contrast agents (Figure 1a). The iNAVs are used to estimate superior-inferior (SI) and right-left (RL) rigid motion by tracking a template around the aortic arch, providing translational motion estimates in a beat-to-beat fashion. SI motion is used to sort the 3D MRA data in equally populated bins, and 3D MR images reconstructed at each respiratory position are used to estimate non-rigid motion between bins (Figure 1b). 2D translational beat-to-beat and 3D non-rigid bin-to-bin motion is then integrated into a motion-compensated patch-based low-rank (PROST) reconstruction8,12 (Figure 1c) to produce the final motion-compensated images.Five healthy subjects and two patients with known cardiovascular disease (4 male, age 34±7 years) were scanned at a 1.5T system (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany) with the proposed non-CE sequence. Data were acquired without contrast agent administration with the following parameters: coronal orientation, 1.6mm3 isotropic resolution, field of view=400x300x128-154mm3, flip angle=90°, T2-preparation duration=40ms, TE/TR=1.72/3.44ms, bandwidth = 930Hz/px, subject-specific mid-diastolic acquisition window, 14 start-up echoes for the 2D iNAVs. For the healthy subjects, acquisition was performed with 3-fold and 5-fold undersampling; and an additional non-CE acquisition with a conventional dNAV gated and motion-tracked sequence (6 mm gating window, tracking scaling factor=0.6, GRAPPA parallel imaging 2x-accelerated, 36 reference lines) was performed with matching imaging parameters for comparison purposes. Acquisitions in patients were performed with the same parameters as in the healthy subjects, with 3-fold undersampling only. As part of the standard care, patients underwent conventional dNAV gated and motion-tracked bSSFP whole-heart imaging ~25 minutes after GBCA administration (Gadovist, 0.15mmol/kg), with the following imaging parameters: sagittal orientation, 1.6mm3 isotropic resolution, field of view=400x155-165x300 mm3, GRAPPA parallel imaging (2x-accelerated, 36 reference lines) and partial Fourier (6/8 slice direction, 6/8 phase encoding direction).
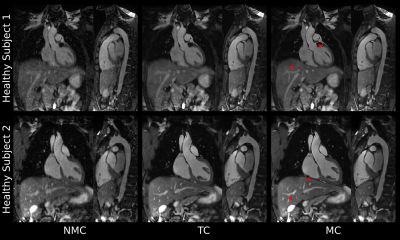
All datasets were reconstructed with the proposed motion-compensated (MC) approach, 2D beat-to-beat translational motion correction only (TC) and without motion correction (NMC) for comparison purposes.
RESULTS
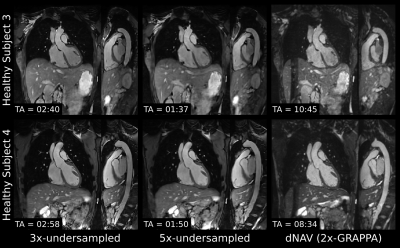
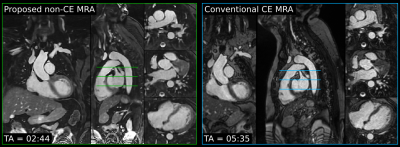
Scans were successfully completed in all subjects, with an average acquisition time for the proposed sequence of 2.9±0.7min for 3-fold undersampling. Translational motion-compensated PROST reconstruction (TC) improves the depiction of the cardiac anatomy and the thoracic vasculature compared to uncorrected images, and further improvements are observed when applying the non-rigid motion-compensated PROST reconstruction (MC), particularly in the abdominal region (Figure 2). The proposed iNAV-based MRA method could be further accelerated without compromising image quality (Figure 3), enabling a scan of 1.7±0.2min (5-fold undersampling), significantly reducing scan time compared to conventional dNAV-based MRA (10.4±1.7min). Furthermore, the non-CE iNAV-based MRA approach produces a depiction of the aorta comparable to conventional CE dNAV-based MRA (Figure 4), but in a shorter and predictable scan time and without the need of exogenous contrast agents. An animation through sagittal reformatting for patient 2 with the proposed non-CE MRA approach is shown in Figure 5.CONCLUSION
We have proposed an efficient protocol for non-CE MRA of the thoracic and suprarenal abdominal aorta. The proposed approach integrates 2D image navigation to enable 100% respiratory scan efficiency and predictable scan time, and an undersampled acquisition and non-rigid motion-compensated reconstruction framework to further accelerate the scan. The approach produces a high-quality depiction of the vessels without exogenous contrast agents from a short scan of ~3 minutes.Acknowledgements
This work was supported by the following grants: BHF 1) PG/18/59/33955, EPSRC 2) EP/P032311/1; 3) EP/P007619/1; 4) EP/P001009/1; and the Wellcome/EPSRC Centre for Medical Engineering (NS/A000049/1).References
1. Hiratzka LF, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients with Thoracic Aortic Disease. J Am Coll Cardiol. 2010;55:e27–129.
2. François CJ, et al. Unenhanced MR angiography of the thoracic aorta: Initial clinical evaluation. Am J Roentgenol. 2008;190:902–6.
3. Amano Y, et al. Non-contrast-enhanced MR angiography of the thoracic aorta using cardiac and navigator-gated magnetization-prepared three-dimensional steady-state free precession. J Magn Reson Imaging. 2008;27:504–9.
4. Von Knobelsdorff-Brenkenhoff F, et al. Comparison of native high-resolution 3D and contrast-enhanced MR angiography for assessing the thoracic aorta. Eur Heart J Cardiovasc Imaging. 2014;15:651–8.
5. Krishnam MS, et al. Image quality and diagnostic accuracy of unenhanced SSFP MR angiography compared with conventional contrast-enhanced MR angiography for the assessment of thoracic aortic diseases. Eur Radiol. 2010;20:1311–20.
6. Haji-Valizadeh H, et al. Accelerated, free-breathing, noncontrast, electrocardiograph-triggered, thoracic MR angiography with stack-of-stars k-space sampling and GRASP reconstruction. Magn Reson Med 2019;81:524–32.
7. Stroud RE, et al. Correcting versus resolving respiratory motion in free-breathing whole-heart MRA: a comparison in patients with thoracic aortic disease. Eur Radiol Exp. 2019;3:29.
8. Bustin A, et al. Highly accelerated 3D whole-heart isotropic sub-Millimeter CMRA with non-rigid motion correction. ISMRM 2019, p 0978.
9. Bustin A, et al. Five-minute whole-heart coronary MRA with sub-millimeter isotropic resolution, 100% respiratory scan efficiency, and 3D-PROST reconstruction. Magn Reson Med. 2019;81:102–15.
10. Prieto C, et al. Highly efficient respiratory motion compensated free-breathing coronary MRA using golden-step Cartesian acquisition. J Magn Reson Imaging. 2015;41:738–46.
11. Henningsson M, et al. Whole-heart coronary MR angiography with 2D self-navigated image reconstruction. Magn Reson Med. 2012;67:437–45.
12. Cruz G, et al. Highly efficient nonrigid motion-corrected 3D whole-heart coronary vessel wall imaging. Magn Reson Med. 2017;77:1894–908.
Figures