2136
Accelerated, free-breathing, contrast-enhanced thoracic MR angiography with XD-GRASP reconstruction1Northwestern University, Chicago, IL, United States, 2Harvard University, Boston, MA, United States
Synopsis
Current methods for contrast-enhanced thoracic MR angiography (CE-MRA) utilize suboptimal imaging practices such as breath holding or long scan time. Here we developed a 9.6-fold accelerated CE-MRA sequence using stack-of-stars k-space sampling and XD-GRASP reconstruction to produce predictable scan time (< 5 min), without significant loss in image quality. Our sequence was significantly faster than the current clinical free-breathing scan, reducing the scan time by 50%. Additionally, our accelerated CE-MRA scan produced comparable image quality that was clinically acceptable.
Introduction
Contrast-enhanced thoracic MR angiography (CE-MRA) is routinely used to diagnose and evaluate aortic diseases including aneurysm, dissection, and aortic valve related aortopathy (1). Two common approaches to thoracic CE-MRA are breath-hold imaging with ECG triggering and inversion-recovery navigator-gated (IR-NAV) CE-MRA. Disadvantages of breath-held ECG-gated CE-MRA include low spatial resolution and spatial coverage, susceptibility to arrhythmia related artifacts, and challenges with breath-holding in some patients. IR-NAV CE-MRA can achieve higher spatial resolution and coverage, but at the expense of scan time (up to 10 min) which is also dependent on patient’s breathing pattern. The purpose of this study was to develop and clinically evaluate a 9.6-fold accelerated IR CE-MRA sequence to produce predictable scan time (< 5 min).Methods
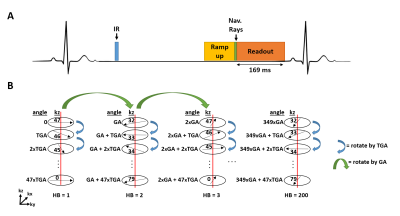
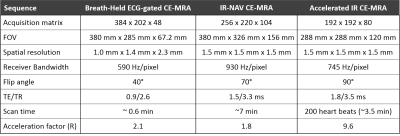
Human Subjects: Twenty patients (15 males, age = 58 ± 17 years) who were undergoing a thoracic MRA for clinically indicated reasons (7 bicuspid aortic valve, 6 aortic aneurysm, 5 aortic dilation, 2 aortic valve replacement) were consented to participate in our study. Patients underwent clinical breath-held ECG-gated CE-MRA (0.15‐0.2 mmol/kg of gadobutrol) and randomized to undergo either the clinical IR-NAV CE-MRA (n=10) or our accelerated IR CE-MRA (n=10).Pulse Sequence: We modified a previously described non-contrast thoracic MRA pulse acquisition with XD-GRASP reconstruction (2) . First, for each shot of 48 rays along the partition direction, rays were rotated with tiny golden angle (TGA) = 23.6281° (3) (Figure 1). Second, the k-space trajectory was designed with a fixed inversion time (TI) to achieve consistent T1 weighting. Relevant imaging parameters for the three CE-MRA sequences used are summarized in Table 1.
Image Reconstruction: XD-GRASP data were reconstructed as previously described (2). Briefly, data were rebinned to 6 respiratory states using principle component analysis of the navigator echo signal. Temporal total variation was used as the sparsifying transform with normalized regularization weight = 0.00075 and fidelity = 0.002 (22 iterations, conjugate gradient with backtracking line search). During post-processing, we applied low rank block wise (4) filtering with 2 iterations using a block size of 8 with a weight of 0.15 and a fidelity weight of 0.01.
Data Analysis: Two cardiovascular radiologists with 8 years and 15 years of clinical experience with MRA graded the image quality. In total, 40 CE-MRA image sets (20 for the breath-held ECG-gated CE-MRA, 10 for the IR-NAV CE-MRA, and 10 for the accelerated IR CE-MRA) from 20 patients were randomized and de-identified for display on a DICOM viewer (RadiAnt DICOM Viewer, Medixant, Poznan, Poland). Prior to visual evaluation, the readers were given training data sets with poor to excellent quality to calibrate reader’s scores in consensus. Following this training session, the readers independently graded the scores by being blinded to image type, each other’s score, and clinical history. The readers graded for each of three categories on a 5-point Likert scale: conspicuity of vasculature (1: nondiagnostic; 2: poor; 3: clinically acceptable; 4: good; 5: excellent), noise and artifact levels (1: nondiagnostic; 2: severe; 3: moderate; 4: mild; 5: minimal). The summed visual scores (SVS) was calculated as the sum of three scores, where 9 was defined as clinically acceptable.
Results
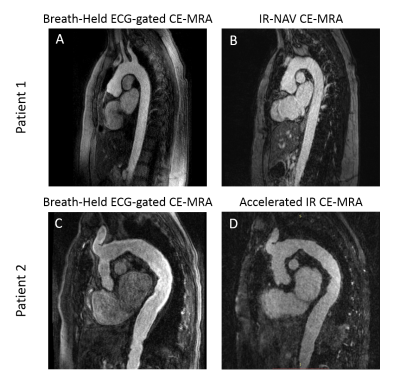
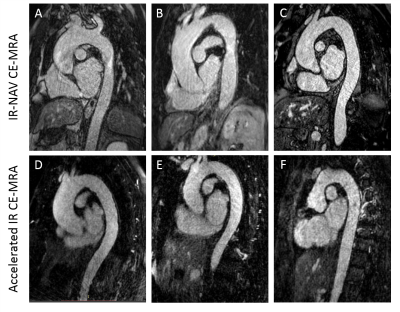
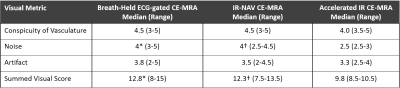
The clinical breath held scan took on average 34 ± 7 seconds and was run immediately after contrast agent administration. The clinical IR-NAV CE-MRA scan was run on average at 2.1 ± 0.8 min after contrast agent administration and took 6.4 ± 2.6 min to complete the scan. Our accelerated IR CE-MRA was run on average 1.3 ± 0.7 min after contrast agent administration and took 3.3 ± 0.5 min to complete the scan. Figure 2 shows representative examples from two patients, illustrating comparable image quality relative to breath-hold CE-MRA as an internal control. Figure 3 shows additional examples from six different patients illustrating image quality between the clinical IR-NAV CE-MRA and our accelerated IR CE-MRA. Upon visual inspection of all 20 patients, the median conspicuity and artifact scores were not significantly different, whereas there were significant differences for noise and SVS (Table 2). Nevertheless, the SVS was greater than 9.0 for all CE-MRA methods.Discussion
Our 9.6-fold accelerated IR CE-MRA was significantly faster than the clinical IR-NAV CE-MRA (p = 0.002) with average scan times of 3.3 ± 0.5 min and 6.4 ± 2.6 min respectively, which represents a ~50% reduction in scan time. Our accelerated IR CE-MRA scan produced comparable image quality that was clinically acceptable. While the accelerated imaging was adequate for the clinical task of visualizing the vasculature, the imaging suffers from higher noise levels compared to the clinical breath-held and clinical IR-NAV scans. One weakness of this study is that the imaging was unpaired between the IR-NAV CE-MRA and the accelerated IR CE-MRA. While it would have been ideal to run both the IR-NAV CE-MRA and the accelerated IR CE-MRA in the same patient, running them back to back inherently reduces the image quality for the second scan run due to gadolinium washout in the blood pool. Therefore, we elected to use the breath-held ECG-gated CE-MRA as an internal control for both sequences.Conclusion
Accelerated IR CE-MRA significantly reduced scan time by half compared to the clinical IR-NAV CE-MRA while maintaining clinically acceptable image quality.Acknowledgements
Funding: National Institutes of Health (R01HL116895, R01HL138578, R21EB024315, R21AG055954, T32EB025766) and American Heart Association (19IPLOI34760317).References
1. Ho VB, Corse WR, Hood MN, Rowedder AM. MRA of the thoracic vessels. Semin Ultrasound CT MR. 2003;24(4):192-216.
2. Haji-Valizadeh H, Collins JD, Aouad PJ, Serhal AM, Lindley MD, Pang J, et al. Accelerated, free-breathing, noncontrast, electrocardiograph-triggered, thoracic MR angiography with stack-of-stars k-space sampling and GRASP reconstruction. Magn Reson Med. 2019;81(1):524-32.
3. Wundrak S, Paul J, Ulrici J, Hell E, Geibel MA, Bernhardt P, et al. Golden ratio sparse MRI using tiny golden angles. Magn Reson Med. 2016;75(6):2372-8.
4. Ong F, Lustig M. Beyond Low Rank + Sparse: Multi-scale Low Rank Matrix Decomposition. IEEE J Sel Top Signal Process. 2016;10(4):672-87.
Figures