2133
Wave-CAIPI Highly Accelerated Whole-Brain Intracranial Vessel Wall Imaging1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2Shenzhen College of Advanced Technology, University of Chinese Academy of Sciences, Shenzhen, China, 3University at Buffalo, The State University of New York, Buffalo, NY, United States
Synopsis
Whole-brain intracranial vessel wall imaging (VWI) requires long scan time for high resolution and large coverage. In this study, we applied a novel three-dimensional parallel imaging technique, Wave-CAIPI, to accelerate whole-brain intracranial VWI. The highly accelerated (11×) VWI takes 3.5 minutes, at an isotropic resolution of 0.6 mm. Compared to conventional two-dimensional parallel imaging technique (2D CAIPI), Wave-CAIPI is able to achieve higher SNR and better imaging quality on vessel wall depictions.
Introduction
Dark-blood MR vessel wall imaging (VWI) is a useful tool in detecting non-stenotic atherosclerosis undetected by bright-blood MR angiography (MRA).1 Intracranial VWI is technically more challenging than carotid VWI,2 since smaller size and deeper location require high resolution and large spatial coverage which lead to long acquisition time. Whole-brain intracranial VWI has been expedited to include the detection of distal branches.3 Though accelerated by partial Fourier, parallel imaging (GRAPPA) and elliptical scanning simultaneously, the acquisition time was still over 7 minutes in recent report.4 A more efficient parallel acquisition technique (2D CAIPI)5 has been demonstrated to achieve better imaging quality than GRAPPA in high-resolution 3D imaging using SPACE sequence.6 However, higher acceleration factor for further scan time reduction is still challenging due to signal-to-noise ratio (SNR) loss in parallel imaging. The recently proposed Wave-CAIPI7 is a novel parallel imaging technique for highly accelerated 3D imaging with improved SNR than 2D CAIPI, by utilizing the coil sensitivity variations in three dimensions. This study aims to apply this technique for highly accelerated (11×) whole-brain intracranial VWI at an isotropic resolution of 0.6 mm, reducing the scan time to 3.5 minutes. It was compared with that accelerated by 2D CAIPI at the same settings in healthy volunteers, in terms of SNR and the quality of vessel wall delineation.Methods
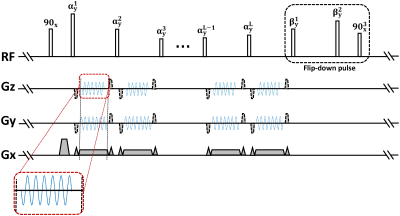
Improved T1-weighted SPACE8 was designed for better cerebrospinal fluid (CSF) suppression in VWI by applying flip-down pulse and modifying the refocusing pulse series. The Wave-CAIPI technique was implemented and applied based on this sequence. Fig. 1 illustrates the sequence diagram. In this work, a slight modification (truncation) was made to the original wave gradients, for keeping zero moment nulling and avoiding the overlaps between the wave gradient and the encoding gradients along Gz. With the modification, the Wave-CAIPI technique can be successfully applied in SPACE sequence for VWI. IRB approved study was performed on a 3T Siemens Tim Trio MRI system with a commercial 32-channel head coil. A young healthy volunteer was recruited with informed consent being obtained. Two whole-brain intracranial VWI scans using the improved T1w-SPACE sequence with different acceleration techniques were performed after localization. One acceleration technique was Wave-CAIPI, and the other was 2D CAIPI for comparison. These two scans used the common parameters as follows: resolution = 0.6 mm, TE/TR = 8.5/1000 ms, bandwidth = 334 Hz/pixel, ETL = 48, FOV = 230 mm (HF, head-foot) × 216 mm (AP, anterior-posterior) × 190 mm (LR, left-right). While in Wave-CAIPI, wave gradient amplitude = 13 mT/m and the maximum slew rate = 192 mT/m/ms, cycles = 7. The acceleration factors of Wave-CAIPI and 2D CAIPI were both 11× (3×3 CAIPIRINHA sampling + elliptical scanning) and both scans took 3.5 minutes. The 2D CAIPI acceleration embedded a 24×24 calibration region in the k-space center for coil sensitivity estimation, while separate ACS data with 24×24 k-space lines was acquired immediately after Wave-CAIPI scan to estimate the coil sensitivity maps. Four calibration scans were also acquired to characterize the point spread function (PSF) for the Wave-CAIPI encoding model. The separate ACS scan and the four calibration scans in Wave-CAIPI took an extra time of about 30 seconds. All datasets were reconstructed offline in MATLAB (Mathworks, Natick, MA, USA) on a workstation with 40 GPU cores and 256 GB memory.Results
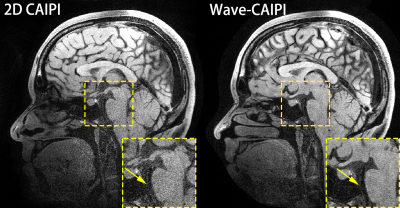
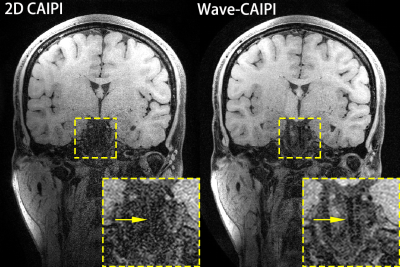
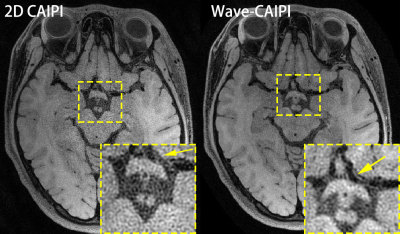
Fig. 2 shows two sagittal slices of the reconstructed 3D images of whole-brain intracranial VWI, which were accelerated by the 2D CAIPI technique and the Wave-CAIPI technique, respectively. The SNR of the reconstructed images in Wave-CAIPI is obviously higher than 2D CAIPI. The vessel walls of the basilar artery (BA) are visible in Wave-CAIPI reconstruction, while deteriorated by the heavy noise in 2D CAIPI reconstruction. This can also be seen in the coronal views (Fig. 3). Fig. 4 presents two transversal slices of the reconstructed 3D images, showing the vessel walls of the internal carotid arteries (ICA). The vessel walls of the ICA are visible in Wave-CAIPI reconstruction, while suffering from severe noise in 2D CAIPI reconstruction. The SNR improvement is more significant in the central part of the field of view (FOV), where the signal sensitivity is reduced due to long distance from the coils. The improvement in such region is valuable, as several intracranial vessels such as basilar artery (BA) and middle cerebral artery (MCA) are deeply located inside the brain.Discussion
In this study, a novel 3D parallel imaging technique, Wave-CAIPI, is introduced to expedite whole-brain intracranial VWI. The highly accelerated (11×) imaging takes 3.5 minutes, at an isotropic resolution of 0.6 mm. Compared to 2D CAIPI, Wave-CAIPI exhibits improved SNR and better quality of vessel wall depictions. The Wave-CAIPI accelerated whole-brain intracranial VWI is still a little bit noisy for clinical acceptance, due to large acceleration and Wave-CAIPI is less beneficial for applications with high resolution and high bandwidth.9 Further improvement could be gained by incorporating sparsity constraint.10 Additionally, recent works have reported the advantages of joint intracranial and carotid vessel wall imaging.11,12 Wave-CAIPI accelerated whole-brain intracranial VWI would be extended to joint intracranial and carotid VWI in the future.Acknowledgements
Zhilang Qiu and Sen Jia contributed equally to this work. This work was supported in part by the grant from the National Natural Science Foundation of China (61871373, 81801691, 81729003 and U1805261), the State Key Program of National Natural Science Foundation of China (81830056), the National Key R&D Program of China (2017YFC0108802), the Strategic Priority Research Program of Chinese Academy of Sciences (XDB25000000), and the Natural Science Foundation of Guangdong Province (2018A0303130132).References
1. Lindenholz A, van der Kolk AG, Zwanenburg JJM, Hendrikse J. The Use and Pitfalls of Intracranial Vessel Wall Imaging: How We Do It. Radiology. 2018;286:12-28.
2. Dieleman N, van der Kolk AG, Zwanenburg JJM, Harteveld AA, Biessels GJ, Luijten PR, Hendrikse J. Imaging Intracranial Vessel Wall Pathology With Magnetic Resonance Imaging Current Prospects and Future Directions. Circulation, 2014;130:192-201.
3. Fan Z, Yang Q, Deng Z, Li Y, Bi X, Song S, Li D. Whole-Brain Intracranial Vessel Wall Imaging at 3 Tesla Using Cerebrospinal Fluid-Attenuated T1-Weighted 3D Turbo Spin Echo. Magn Reson Med. 2017;77:1142-1150.
4. Yang Q, Deng Z, Bi X, Song S, Schlick KH, Gonzalez NR, Li D, Fan Z. Whole-brain vessel wall MRI: A parameter tune-up solution to improve the scan efficiency of three-dimensional variable flip-angle turbo spin-echo. J Magn Reson Imaging. 2017; 46:751-757.
5. Breuer FA, Blaimer M, Mueller MF, et al. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn Reson Med. 2006;55:549–556.
6. Fritz J, Fritz B, Thawait GG, Meyer H, Gilson WD, Raithel E. Three-Dimensional CAIPIRINHA SPACE TSE for 5-Minute High-Resolution MRI of the Knee. Invest Radiol. 2016; 51, no. 10:609-17.
7. Bilgic B, Gagoski BA, Cauley SF, et al. Wave-CAIPI for highly accelerated 3D imaging. Magn Reson Med. 2015;73:2152–2162.
8. Zhang L, Zhang N, Wu J, Liu X, Chung YC. High resolution simultaneous imaging of intracranial and extracranial arterial wall with improved cerebrospinal fluid suppression. Magn Reson Imaging. 2017;44:65-71.
9. Wang H, Qiu Z, Su S, Jia S, Li Y, Liu X, Zheng H, Liang D. Parameter optimization framework on wave gradients of Wave-CAIPI imaging. Magn Reson Med. 2019; DOI: 10.1002/mrm.28034.
10. Curtis AT, B. Bilgic, K. Setsompop, R. S. Menon, and C. K. Anand, "Wave-CS: Combining wave encoding and compressed sensing," In Proceedings of the 23th Annual Meeting of ISMRM, Toronto, Canada, 2015. p. 82.
11. Zhang L, Zhang N, Wu J, Liu X, Chung YC. High resolution simultaneous imaging of intracranial and extracranial arterial wall with improved cerebrospinal fluid suppression," Magn Reson Imaging, vol. 44, pp. 65-71, Dec 2017.
12. Jia S, Zhang L, Ren L, Qi Y, Ly J, Zhang N, Li Y, Liu X, Zheng H, Liang D, Chung YC. Joint intracranial and carotid vessel wall imaging in 5 minutes using compressed sensing accelerated DANTE-SPACE. Eur Radiol. 2019; DOI: 10.1007/s00330-019-06366-7.
Figures