2132
Diffusion-Prepared SPACE sequence: Application to Isotropic 3D T2W Black Blood MRI of the Whole Aortic Wall1Siemens Magnetic Resonance Ltd., Shenzhen, China
Synopsis
T2-weighted MR imaging is a non-invasive way for vasculitis
diagnosis. SPACE sequence has been successfully used for T1-weighted aortic
wall imaging. While its application in T2w aortic wall imaging
is hampered by the long scanning time, blood suppression performance
and physiological movement. In this study, three black blood methods with
optimized protocols have been tested and evaluated. Diffusion-Prepared SPACE
sequence with diastolic acquisition was proved to be a promising way for T2w
aortic wall imaging within a clinically acceptable acquisition time.
Introduction
Vasculitis, the inflammation of blood vessels, is rare [1], yet can affect people of all ages and sometimes can be very serious. T2-weighted (T2W) MR imaging provided a non-invasive way to detect and diagnose the vasculitis. The conventional way for T2W aortic wall imaging is T2W DIR-prepared 2D fast spin-echo [2]. However, it is susceptible to partial volume effects and compromises aortic coverage when sections are positioned perpendicular to the direction of aortic blood flow. High resolution, Isotropic 3D fast spin echo sequence with variable flip angle (SPACE) is advantageous in aortic wall imaging. It has been successfully used for T1-weighted (T1W) aortic wall imaging in healthy volunteers and patients with cardiovascular or atherosclerotic disease with high isotropic resolution [3-6]. However, it is not widely used for T2W aortic wall imaging because of the long scanning time. Other challenges like insufficient blood suppression, motion from heartbeat and respiration are also critical for aortic wall imaging. In this study, we aim to implement three blood suppression methods and evaluate the performance in healthy volunteers and optimize the protocols to achieve good image quality within a clinically acceptable scan time.Methods
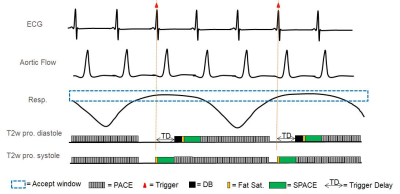
SPACE sequence has its intrinsic black blood effect, however, blood suppression is less effective in the presence of slow or complex flow, and flow artifacts have been observed even in healthy volunteers with normal aorta geometry [2]. Three methods have been provided to further suppress the blood signal: (1) Diffusion-Prepared pulse (2) DANTE preparation pulse (3) Data acquisition during systole to enhance its intrinsic black blood effect. 2D navigator-triggered prospective acquisition correction (2D-PACE) [7] technique and cardiac triggering have been used to alleviate the aortic motion. Acquisition was triggered every respiratory cycle. Therefore, the real TR was equal to one respiratory cycle (3-4s). Pulse sequence diagram of SPACE sequence with combined 2D PACE and ECG trigger is shown in Fig. 1. The other basic parameters of T2w SPACE sequence were as follows: TE = 106ms; echo train length = 135; FOV = 300mm×400mm; matrix size = 240×320; bandwidth = 820 Hz/pixel; 56 sagittal slices with 1.3mm slice thickness; spectrally selective fat saturation was used, parallel imaging (GRAPPA) acceleration factor = 2, average = 1.8; The total acquisition time was around 5.5min (5+75 respiratory cycle); 1.3mm isotropic resolution was achieved.Results
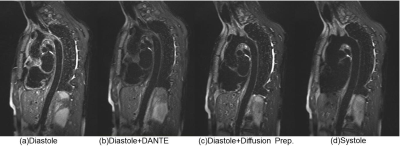
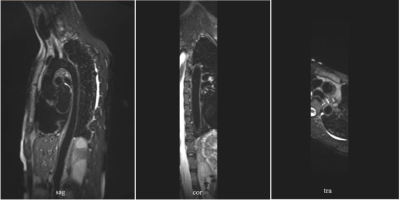
In compliance with IRB requirements, one healthy adult volunteer was scanned on 1.5T MRI system (MAGNETOM Amira, Siemens Healthcare, Erlangen, Germany). Four measurements have been performed as the following: (1) Diastolic acquisition without black blood preparation pulse. (2) Diastolic acquisition with DANTE preparation pulse. (3) Diastolic acquisition with diffusion-prepared pulse. (4) Systolic acquisition without black blood preparation pulse, fast flow during systole can enhance SPACE intrinsic black blood effect. A comparison of sample images results from SPACE with different black blood techniques is shown in Fig. 2. During diastole, the blood flow is slow and complex flow exists in aortic arch. Therefore the intrinsic black blood effect is not enough. Flow artifacts can be observed in aortic arch in Fig. 2(a). Fig. 2(b) shows the addition of DANTE removed flow artifacts partially, while the wall signal is also decreased significantly. Fig. 2(c) shows the addition of diffusion-prepared pulse reduced the flow artifacts substantially and the wall signal is mostly remained. Fig. 2(d) shows the data acquisition during systole can remove the flow artifacts completely. However, the wall signal is also influenced by the pulsation of the aorta. Diffusion-Prepared SPACE with diastolic acquisition was found to be most promising for T2W whole aortic wall imaging. Images in sagittal, coronal and transversal plane are displayed in Fig. 3.Conclusion
This study optimizes a high resolution, 3D MRI for T2w Aortic wall imaging in a clinically acceptable scan time. The diffusion preparation module substantially reduces flow artifacts that are otherwise noted on SPACE in health volunteer. Diffusion-Prepared SPACE shows promise as a non-invasive tool for vasculitis diagnosis.Acknowledgements
References
1. Gonzalez-Gay MA, Garcia-Porrua C: Epidemiology of the vasculitides. Rheum Dis Clin North Am 2001, 27:729–749.
2. Ioannis K, Debiao Li. Diffusion-Prepared Segmented Steady-State Free Precession: Application to 3D Black-Blood Cardiovascular Magnetic Resonance of the Thoracic Aorta and Carotid Artery Walls. Journal of Cardiovascular Magnetic Resonance (2007) 9, 33–42.
3. Mihai G, Chung YC, Merchant A, Simonetti OP, Rajagopalan S. T1-weighted-SPACE dark blood whole body magnetic resonance angiography (DB-WBMRA): initial experience. J Magn Reson Imaging. 2010; 31:502–509.
4. Mihai G, Varghese J, Lu B, Zhu H, Simonetti OP, Rajagopalan S. Reproducibility of thoracic and abdominal aortic wall measurements with three-dimensional, variable flip angle (SPACE) MRI. J Magn Reson Imaging. 2013
5. Wong SK, Mobolaji-Iawal M, Arama L, Cambe J, Biso S, Alie N, Fayad ZA, Mani V. Atherosclerosis imaging using 3D black blood TSE SPACE vs 2D TSE. World J Radiol. 2014; 6:192–202.
6. Chengcheng Z, Henrik H, Farshid F. Isotropic 3D Black Blood MRI of Abdominal Aortic Aneurysm Wall and Intraluminal Thrombus. Magn Reson Imaging. 2016 January ; 34(1): 18–25.
7. Asbach P et al. Magnetic resonance cholangiopancreatography using a free-breathing T2-weighted turbo spin-echo sequence with navigator triggered prospective acquisition correction. Magn Reson Imaging 2005; 23: 939 –945.