2130
4-minute 3D Whole Brain 0.6mm Isotropic T1 Weighted Intracranial Vessel Wall Imaging1Philips Research North America, Cambridge, MA, United States, 2Department of Radiology, University of Washington, Seattle, WA, United States, 3Philips Research Hamburg, Hamburg, Germany
Synopsis
Three-dimensional (3D) T1 weighted (T1w) turbo spin echo (TSE) sequence has demonstrated its value for intracranial vessel wall imaging (IVWI), but its long scan time has hampered wider clinical translation. In this study, a 4-minute 3D T1w TSE sequence was optimized and combined with wave encoded parallel imaging and compressed sensing (PI-CS) reconstruction for whole brain isotropic 0.6mm IVWI. This approach improved the image quality and robustness of IVWI in comparison to the Cartesian encoded PI-CS approach in healthy subjects. It demonstrated promising potential for further clinical evaluation.
Introduction
Three-dimensional (3D) T1 weighted (T1w) turbo spin echo (TSE) sequence with variable flip angle (VFA) refocusing pulse train has become the most preferable technique for whole brain high resolution intracranial vessel wall imaging (IVWI) at 3T, where various optimizations have been applied to improve signal-to-noise ratio (SNR), point spread function (PSF) and image contrast1-5. However, its wider clinical translation remains hampered by the long scan time. Some recent studies6,7 have demonstrated that parallel imaging and compressed sensing (PI-CS) can effectively reduce the IVWI scan time to 5~6 minutes. In addition, wave encoding8 as a subsampled artifacts smearing technique has shown its improved PI performance by more properly using the entire 3D spatial encoding power of multi-channel coil sensitivities. In this study, the 3D T1w TSE sequence is further optimized and accelerated with the wave encoded PI-CS to achieve whole brain 0.6mm isotropic IVWI in 4 minutes. This approach is compared with the Cartesian encoded PI-CS and its feasibility is demonstrated by healthy volunteer scans.Methods
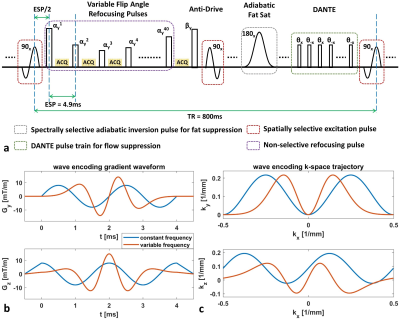
Sequence OptimizationThe sequence diagram of optimized T1w 3D TSE is shown in figure 1. To improve the SNR, a VFA design strategy to achieve low-pass filtering shaped PSF profile was exploited and the echo time was minimized without skipping any TSE echoes9,10. DANTE pre-pulse11,12 was applied to improve flow suppression. Also, the anti-drive tip-down pulse3,4 was used at the end of TSE pulse train to improve T1 contrast. The data acquisition of this sequence was implemented with Cartesian and wave encoding separately for comparison. For wave encoding, a variable frequency wave encoding scheme13 (figure 1b&c) was performed to improve the aliasing propagation property along the readout direction, while reducing the eddy current induced slice profile imperfection.
Image Reconstruction
The central k-space area of 20x20 was fully sampled to calibrate the coil sensitivities (and wave PSF for wave encoding). A recently developed subspace-based wave encoding self-calibration approach14 was used with an additional k-space trajectory symmetric constraint to stabilize the wave PSF calibration. Coil sensitivities were estimated by an auto-calibration method15,16. The L1-wavelet regularized SENSE framework was used to reconstruct the image: $$\min_{x}\frac{1}{2}\parallel{y-PF_{yz}PsfF_{x}Sx}\parallel_2^2+\lambda\parallel{Wx}\parallel_{1}.$$ Here $$$x$$$ is the to-be-reconstructed image, $$$y$$$ represents the acquired k-space samples, $$$F_{x}/F_{yz}$$$ represents the Fourier transform operator along x direction or within y-z plane, $$$S$$$ is the sensitivity map, $$$Psf$$$ stands for the wave PSF (an identity matrix for Cartesian encoding), $$$P$$$ denotes the undersampling mask, $$$W$$$ indicates the wavelet transform, and $$$\lambda$$$ is the regularization weight (set to 0.1 in this work). Coil compression17 was applied before the calibration and reconstruction to improve the overall computational efficiency with 9 virtual coils.
MR scans
The Cartesian and wave encoded sagittal 3D T1w TSE datasets were acquired by a 32-channel head coil on a Philips Ingenia 3.0T scanner using the following parameters: FOV (FHxAPxRL) = 230x230x200mm3, resolution = 0.6x0.6x0.6mm3, TE/TR = 4.9ms/800ms, TSE factor = 40, 100 of 1.5ms DANTE units with flip angle of 12deg, adiabatic spectral fat suppression. A poisson-disc variable density random sampling was prospectively applied to accelerate the scan by 8.5 fold and achieve a 4-minute IVWI protocol. For wave encoding, a maximum wave encoding gradient strength of 15mT/m was used to improve the aliasing propagation along the readout direction, while decreasing the number of cycles to 2 for slew rate reduction.
Results
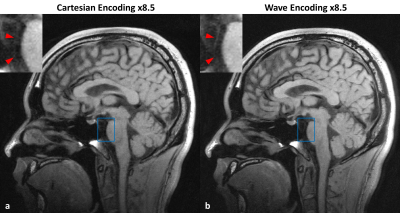
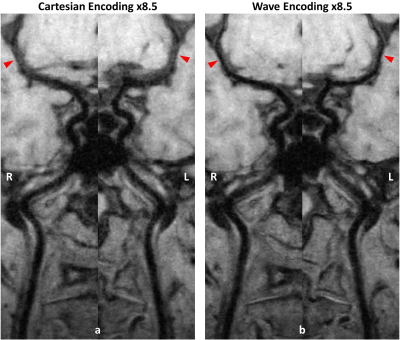
Figure 2 shows one example comparing reconstructed images between Cartesian and wave encoding. Wave encoding provided overall less noise amplification and improved image quality at such a high acceleration factor scenario. In particular, the basilar artery vessel wall can be better preserved on the wave encoded reconstruction result as shown by the zoom-in regions. Figure 3 compares the curved planar reconstruction images of bilateral cerebral arteries between Cartesian and wave encoded reconstruction results. Both imaging methods showed sufficient flow suppression from internal carotid arteries to middle cerebral arteries. With wave encoding, the vessel wall of middle cerebral arteries can be more stably delineated.Discussion and Conclusion
Wave encoding enables additional improvement of PI-CS so that the small and deep intracranial vessels can be more accurately and stably restored in highly accelerated IVWI. To rapidly reconstruct such huge datasets (high resolution, large coverage, and many coil channels), coil compression would be critical to reduce memory and time consumption, which however may limit the PI performance. This experiment demonstrated that wave encoding can mitigate such limitation by taking better usage of the entire 3D coil sensitivity encoding power. This 4-minute 3D T1w whole brain 0.6mm isotropic IVWI with wave encoded PI-CS reconstruction has demonstrated promising results in healthy subjects. Further evaluation on patients is warranted for clinical translation.Acknowledgements
This study was supported in part by the grant from National Institutes of Health (R01NS092207).References
1. Qiao Y, Steinman DA, Qin Q, Etesami M, Schär M, Astor BC, Wasserman BA. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging. 2011;34(1):22-30.
2. Qiao Y, Steinman DA, Qin Q, Etesami M, Schär M, Astor BC, Wasserman BA. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology. 2014;271(2):534-42.
3. Yang H, Zhang X, Qin Q, Liu L, Wasserman BA, Qiao Y. Improved cerebrospinal fluid suppression for intracranial vessel wall MRI. J Magn Reson Imaging. 2016;44(3):665-72.
4. Fan Z, Yang Q, Deng Z, Li Y, Bi X, Song S, Li D. Whole-brain intracranial vessel wall imaging at 3 Tesla using cerebrospinal fluid-attenuated T1-weighted 3D turbo spin echo. Magn Reson Med. 2017;77(3):1142-1150.
5. Yang Q, Deng Z, Bi X, Song SS, Schlick KH, Gonzalez NR, Li D, Fan Z. Whole-brain vessel wall MRI: A parameter tune-up solution to improve the scan efficiency of three-dimensional variable flip-angle turbo spin-echo. J Magn Reson Imaging. 2017;46(3):751-757.
6. Zhu C, Tian B, Chen L, Eisenmenger L, Raithel E, Forman C, Ahn S, Laub G, Liu Q, Lu J, Liu J, Hess C, Saloner D. Accelerated whole brain intracranial vessel wall imaging using black blood fast spin echo with compressed sensing (CS-SPACE). MAGMA. 2018;31(3):457-467.
7. Jia S, Zhang L, Ren L, Qi Y, Ly J, Zhang N, Li Y, Liu X, Zheng H, Liang D, Chung YC. Joint intracranial and carotid vessel wall imaging in 5 minutes using compressed sensing accelerated DANTE-SPACE. Eur Radiol. 2019 Aug 1. Epub.
8. Bilgic B, Gagoski BA, Cauley SF, Fan AP, Polimeni JR, Grant PE, Wald LL, Setsompop K. Wave-CAIPI for highly accelerated 3D imaging. Magn Reson Med. 2015;73(6):2152-62.
9. Zhou Z, Chen S, Wu J, Börnert P, Yuan C. Deep Convolutional Neural Network Enhanced 3D High Resolution Turbo Spin Echo Intracranial Vessel Wall Imaging. ISMRM 2018, p1049.
10. Zhou Z, Chu B, Sun J, Balu N, Mossa-basha M, Hatsukami TS, Börnert P, Yuan C. Highly Accelerated Whole Brain Isotropic 0.5mm Intracranial Vessel Wall Imaging with Nonlocal Denoising and Super Resolution Enhancement. ISMRM 2019, p3242.
11. Li L, Miller KL, Jezzard P. DANTE-prepared pulse trains: a novel approach to motion-sensitized and motion-suppressed quantitative magnetic resonance imaging. Magn Reson Med. 2012;68(5):1423-38.
12. Wang J, Helle M, Zhou Z, Börnert P, Hatsukami TS, Yuan C. Joint blood and cerebrospinal fluid suppression for intracranial vessel wall MRI. Magn Reson Med. 2016;75(2):831-8.
13. Zhou Z, Chu B, Yuan C, Börnert P. Variable frequency wave-encoded 3D turbo spin echo imaging. ISMRM 2019, p4577.
14. Zhou Z, Yuan C, Börnert P. Self-calibrating wave-encoded 3D turbo spin echo imaging using subspace model based autofocusing. Magn Reson Med. 2019 Oct 19. Epub.
15. Ying L, Sheng J. Joint image reconstruction and sensitivity estimation in SENSE (JSENSE). Magn Reson Med. 2007;57(6):1196-202.
16. Uecker M, Hohage T, Block KT, Frahm J. Image reconstruction by regularized nonlinear inversion-joint estimation of coil sensitivities and image content. Magn Reson Med. 2008;60(3):674-82.
17. Zhang T, Pauly JM, Vasanawala SS, Lustig M. Coil compression for accelerated imaging with Cartesian sampling. Magn Reson Med. 2013;69(2):571-82.
Figures