2125
Accurate detection of calcified carotid plaques on quantitative susceptibility mapping – A CT validation study1Weill Cornell Medicine, New York, NY, United States, 2Xuanwu Hospital of Capital Medical University, Beijing, China
Synopsis
In this study, we used CT angiography (CTA) as a reference standard to demonstrate that carotid quantitative susceptibility mapping (QSM) provides accurate detection of calcified atherosclerotic plaques in the carotid arteries. Calcification is strongly diamagnetic and appears uniquely hypointense on carotid QSM. Inclusion of QSM in carotid MRI would aid characterization of carotid plaque.
INTRODUCTION
Calcification is a common feature of carotid atherosclerotic plaques, which indicates plaque stability and may reduce stroke risk in half (1). Heavily calcified plaques are associated with less favorable outcomes of carotid revascularization. Therefore, accurate assessment of plaque calcification is critically important to triaging patients for medication vs. revascularization intervention. CT angiography (CTA), currently the gold standard for in vivo calcification detection, requires harmful radiation and a large dose of iodine contrast agent but does not provide characterization of other important intraplaque components including hemorrhage of high risk. Multi-contrast MRI (mcMRI), useful for characterizing intraplaque components without CTA limitations (2), allows detection of calcification based on hypointensity on T1w/T2w images. However, accurate interpretation of calcification on mcMRI is difficult, as hemosiderin-rich intraplaque hemorrhage (IPH), perivascular fat, and blood vessels also appear hypointense on the black blood fat-suppressed mcMRI images. This mcMRI ambiguity can be resolved on quantitative susceptibility mapping (QSM), because calcification is a uniquely strong diamagnetic source in the body (3-5) and is easily distinguishable from paramagnetic sources such as hemorrhage and fat. The objective of this study was to evaluate the improvement in diagnostic accuracy of calcification detection in carotid plaques by adding QSM to mcMRI, using CTA as the reference standard.METHODS
Carotid plaque mcMRI. The clinical imaging protocol was based on ASNR Vessel Wall Imaging Study Group recommendations (6) and consisted of 3D TOF, 2D black blood fat-suppressed T1w/T2w TSE, and 3D magnetization-prepared rapid gradient echo (MPRAGE) sequences with spatial resolution of 0.6x0.6x2 mm3 and 6 cm longitudinal coverage of the carotid bifurcation.Carotid QSM. A multi-echo 3D GRE sequence was optimized for carotid QSM to match the spatial resolution and coverage of mcMRI with 5 min scan time. Four echo times were acquired per TR with 4.7 ms in-phase echo spacing to allow optimal field estimation in the presence of fat at 3T (7). A nonlinear preconditioned total field inversion algorithm was developed to calculate the susceptibility map by minimizing the following cost function:
$$x^*=arg min_x{\parallel}w(e^{-i\alpha f}-e^{-id*Px}){\parallel}^2_2+{\lambda\parallel}M_G{\triangledown}Px{\parallel}_1+{\lambda_{A}\parallel}M_{A}P(x-\overline{x}^{A}){\parallel}^2_2$$
Here the first two terms are the data fidelity term and structure consistency regularization term, where $$$w$$$ is the SNR weighting, $$$d$$$ is the dipole kernel, $$$M_G$$$ is the edge mask, and $$$\triangledown$$$ is the gradient operator. The third term enforces susceptibility homogeneity of the well-mixed arterial blood (8), $$$M_{A}$$$ is the arterial mask (obtained using a semi-automated region-growing algorithm), and $$$\overline{x}^{A}$$$ is the mean susceptibility within the mask. $$$\alpha$$$ is a scalar that scales down $$$f$$$ to avoid phase wraps. The preconditioner $$$P$$$ is used to accelerate convergence (9). The final susceptibility map is $$$Px^*/\alpha$$$.
Imaging study. Ten patients (mean age 66 years ± 5) had both MRI and CTA scans with median follow-up interval of 3 days. A neuroradiologist with 13 years of carotid imaging experience independently reviewed CTA and mcMRI (without and with QSM image) to identify plaque calcification on a per vessel basis. Calcification was detected as region with high attenuation on CTA (Hounsfield unit>100), hypointense signal (compared to the adjacent muscle) on mcMRI, and strongly negative susceptibility (<-0.5 ppm) on QSM.
RESULTS
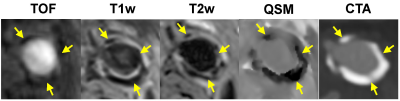
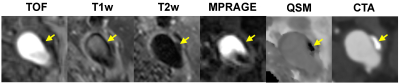
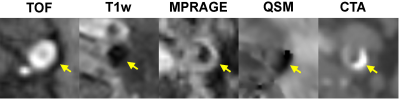
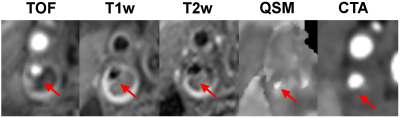
Figure 1 shows an example of concordant depiction of a heavily calcified plaque at the carotid bifurcation on mcMRI, QSM and CTA images. Compared to mcMRI, the unambiguous visual contrast between calcification and the surrounding tissues on QSM allows easy identification and improves diagnostic confidence. This benefit is highlighted in Figs. 2 and 3, which show examples of small calcification nodules with dark signal that could not be prospectively identified by the experienced reader on mcMRI but well captured by QSM (approximately -2 ppm susceptibility) in excellent agreement with CTA. Out of 17 vessels with calcified plaques detected by CTA, mcMRI only captured 11 calcifications (64.7% sensitivity). However, with the addition of QSM, all calcifications were identified (100% sensitivity). Both mcMRI and QSM correctly identified the 3 non-calcified vessels (100% specificity). Additionally, Figure 4 demonstrates the versatility of QSM for detecting paramagnetic intraplaque hemorrhage consistent with low attenuation (Hounsfield unit <60) on CTA image.DISCUSSION
Our preliminary results suggest that QSM substantially improves the diagnostic accuracy for detecting calcified carotid plaques. Our QSM validation study with CTA is ongoing, and the patient cohort size will be significantly increased in the future. QSM has the potential to become an instrumental part of noninvasive and quantitative carotid MRI for plaque characterization. QSM is known to provide sensitive detection of strong magnetic materials in tissue, including highly paramagnetic iron in hemorrhage and highly diamagnetic calcification deposits. Accordingly, QSM can reliably resolve the ambiguity of T1w hypointensity on traditional mcMRI, which can be the result of IPH and/or calcification. Future clinical studies with large patient sample sizes are warranted to establish the value of including QSM in carotid MRI protocol, which would enable confident triaging carotid patients who are at high risk and would benefit from surgical interventions to prevent stroke, from those with lower risk who would derive a similar risk reduction benefit from noninvasive medication treatment.Acknowledgements
No acknowledgement found.References
1. Baradaran
H, Al-Dasuqi K, Knight-Greenfield A, Giambrone A, Delgado D, Ebani EJ, Kamel H,
Gupta A. Association between carotid plaque features on CTA and cerebrovascular
ischemia: A systematic review and meta-analysis. AJNR Am J Neuroradiol. 2017;38(12):2321-6.
2. Cai
JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of
human carotid atherosclerotic lesions with in vivo multicontrast magnetic
resonance imaging. Circulation 2002;106(11):1368-73.
3. Ikebe
Y, Ishimaru H, Imai H, Abe K, Izumo T, Morofuji Y, Ideguchi R, Morikawa M,
Uetani M. Quantitative susceptibility mapping for carotid atherosclerotic
plaques: A pilot study. Magn Reson Med Sci 2019 May 31.
4. Wang
C, Liu S, Chen Y, Buch S, Fan Z, Haacke EM, Yang Q. Intraplaque hemorrhage and
calcification detection with quantitative susceptibility mapping. ISMRM
2018;491.
5. Chen
W, Zhu W, Kovanlikaya I, Kovanlikaya A, Liu T, Wang S, Salustri C, Wang Y.
Intracranial calcifications and hemorrhages: characterization with quantitative
susceptibility mapping. Radiology 2014;270(2):496-505.
6. Saba
L, Yuan C, Hatsukami TS, Balu N, Qiao Y, DeMarco JK, Saam T, Moody AR, Li D,
Matouk CC, Johnson MH, Jager HR, Mossa-Basha M, Kooi ME, Fan Z, Saloner D,
Wintermark M, Mikulis DJ, Wasserman BA, Vessel Wall Imaging Study Group of the
American Society of N. Carotid Artery Wall Imaging: Perspective and Guidelines
from the ASNR Vessel Wall Imaging Study Group and Expert Consensus
Recommendations of the American Society of Neuroradiology. AJNR Am J
Neuroradiol 2018;39(2):E9-E31.
7. Pineda
AR, Reeder SB, Wen Z, Pelc Cramér-Rao bounds for three-point decomposition of
water and fat. Magn Reson Med 2005;54(3):625-35.
8. Liu
Z, Spincemaille P, Yao Y, Zhang Y, Wang Y. MEDI+0: Morphology enabled dipole
inversion with automatic uniform cerebrospinal fluid zero reference for
quantitative susceptibility mapping. Magn Reson Med
2018;79(5):2795-803.
9. Liu
Z, Kee Y, Zhou D, Wang Y, Spincemaille P. Preconditioned total field inversion
(TFI) method for quantitative susceptibility mapping. Magn Reson Med 2017;78(1):303-15.
Figures