2122
Time-efficient large field-of-view fat suppression using SNR priority Dixon turbo spin echo for femoral vessel wall MRI1Radiology, University of Washington, Seattle, WA, United States, 2Philips Healthcare, Briarcliff Manor, NY, United States, 3Surgery, University of Washington, Seattle, WA, United States
Synopsis
Vessel wall MRI requires good fat suppression to delineate
outer vessel boundaries. Large field-of-views required for turbo spin echo
femoral vessel wall MRI do not provide good fat suppression with spectral inversion
recovery. We developed a time-efficient two-point Dixon turbo spin echo method
using a SNR priority variable flip angle schedule and point spread correction
reconstruction to allow accelerated acquisition and demonstrate its advantages
compared to an existing gradient echo vessel wall MRI method.
Introduction
Large coverage isotropic resolution vessel wall MRI (VWI) is challenging due to competing requirements of scan time and signal-to-noise ratio (SNR). While variable flip angle turbo spin echo MRI (VFA-TSE) sequences provide high SNR, they do not provide effective or uniform fat suppression which is critical to distinguish the outer boundary of the vessel wall. VFA-TSE fat suppression tends to worsen further for large field-of-view (FOV) VWI such as femoral artery VWI. While VFA-TSE has been demonstrated for VWI applications, their use has been confined to either intracranial VWI [1] which does not require fat suppression or smaller FOV VWI such as carotid VWI [2] or their combination [3,4]. Fat suppression for femoral VWI is particularly challenging since it requires a large FOV starting from the groin to below the knees. Previous femoral VWI methods have therefore used gradient echo-based sequences [5,6] which provide sufficient (but non-uniform) fat suppression. While these gradient echo-based methods are also time-efficient, they are highly susceptible to B0 inhomogeneity and do not provide uniform vessel signal over the large FOV.Aim
To develop a time efficient fat suppressed large FOV VFA-TSE VWI sequence covering the femoral arteries from above the femoral bifurcation to below the knees.Methods
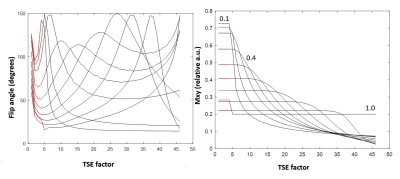
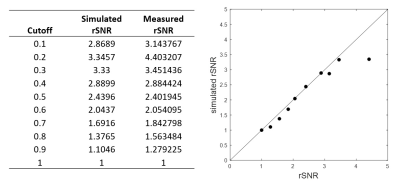
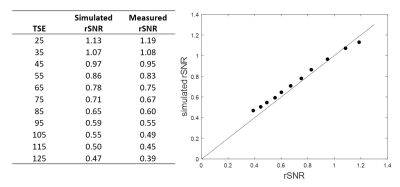
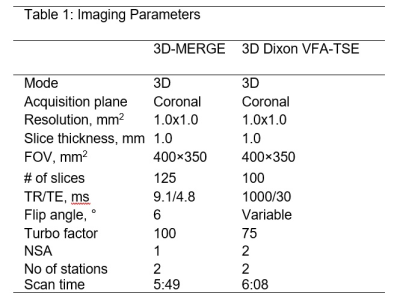
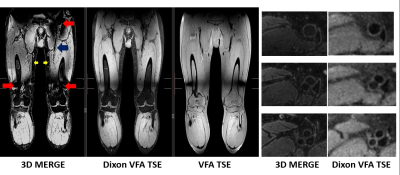
A SNR priority variable flip angle schedule [7] was optimized by Bloch-simulation (Simulation parameters: T1:844ms,T2:39ms,TSE:42,Startup echoes:4,TR:1000ms) to determine the optimum VFA k-space filter cutoff-frequency that provides higher signal than VFA-TSE with the sharpest possible point-spread function (PSF). A k-space filter cutoff of 0.4 is optimal by this criterion. Figure 1 shows the simulated VFA profiles and figure 2 shows signal relative to a filter cutoff of 1.0 (highest possible PSF but lowest signal). With cutoff held at 0.4, the effect of increasing TSE factor was Bloch-simulated to determine the maximum possible acceleration possible using large turbo factors (figure 3). SNR measurements were performed using a uniform bottle phantom and separate noise scan (no RF pulse) to confirm simulation predictions. All scans were conducted on a Philips Ingenia 3T CX whole body scanner under IRB approved guidelines. Using optimized parameters from Bloch simulation, VFA-TSE and 3D-MERGE [5] scans were then compared in vivo as follows: VFA-TSE with two-point Dixon fat-suppression [8] and similar scan time as 3D-MERGE was scanned in two stations (40cm S/I FOV each) with a 5cm overlap between stations. In vivo imaging parameters were optimized on one volunteer and are shown in table 1. Dixon VFA-TSE, 3D-MERGE and VFA-TSE with spectral adiabatic inversion recovery fat suppression (SPAIR) were compared in a volunteer. In vivo SNR priority VFA-TSE scans were PSF corrected using a PSF correction neural network [7]. Fat suppression, outer wall visibility and SNR were compared between VFA-TSE and 3D-MERGE. Muscle signal, fat signal in adjacent region and lumen signal were measured in the thigh. Noise was measured as the standard deviation of muscle signal to account for use of phased array coils. Muscle-fat contrast-to-noise ratio (CNRmf) and muscle-lumen CNR (CNRml) were also compared.Results
A k-space filter cutoff of 0.4 was predicted to provide 2.88x times the signal relative to cutoff of 1.0 and was used as the optimum cutoff for in vivo scans. SNR measurements on phantom confirmed simulation predictions. Increasing TSE was predicted to reduce SNR but not impact PSF. SNR measurement on a phantom using VFA-TSE and 3D-MERGE showed that TSE factors up to 85 can be used without SNR penalty relative to 3D-MERGE. In vivo optimization scans showed that four startup echoes provided sufficient blood suppression. A TSE factor 75 provided similar scan time as 3D-MERGE. 2X SENSE acceleration was additionally used for Dixon VFA-TSE only. Two-point Dixon provided uniform fat suppression for accelerated VFA-TSE across the entire FOV (figure 4) visually comparable to 3D-MERGE. Signal homogeneity was substantially better on Dixon VFA-TSE compared to 3D-MERGE, which suffered from flame artifacts and overall reduced SNR at the edges of the FOV.Muscle SNR was substantially higher on Dixon VFA-TSE compared to 3D-MERGE (33.6±5.4 vs 14.2±3.9, p<0.05). CNRmf was similar on Dixon VFA-TSE and 3D-MERGE (0.5±0.1 vs 0.1±0.4, p=0.16) showing that fat suppression was adequate on Dixon VFA-TSE for identifying vessel wall. However, the much lower standard deviation of Dixon VFA-TSE compared to 3D-MERGE suggests uniform fat suppression on Dixon VFA-TSE while 3D-MERGE fat suppression was non-uniform. CNRmf was higher on Dixon VFA-TSE than 3D-MERGE (0.7±0.0 vs 0.5±0.0, p<0.05) suggesting better blood suppression than 3D-MERGE. Compared to SPAIR VFA-TSE, Dixon VFA-TSE had substantially higher CNRmf (0.7±0.0 vs 0.0±0.14, p<0.05) showing that fat suppression was significantly improved with Dixon VFA-TSE.
Conclusions
A novel acceleration method using long flip angle trains of SNR priority VFA TSE flip angle schedules and PSF correction combined with two-point Dixon fat suppression allows time-efficient large coverage peripheral artery vessel wall MRI. Dixon VFA-TSE provides higher SNR, more uniform fat suppression and better flow suppression than 3D-MERGE. Thus, Dixon VFA-TSE method is a promising method for large coverage vessel wall imaging and may find applications in abdominal and whole body VWI applications in addition to femoral VWI.Acknowledgements
References
[1] Qiao Y, Steinman DA, Qin Q, Etesami M, Schar M, Astro BA, Wasserman BA. Intracranial Arterial Wall Imaging using 3D High Isotropic Resolution Black Blood MRI at 3.0 T. J Magn Reson Imaging. 2011; 34(1):22-30.
[2] Fan Z, Zhang Z, Chung YC, Weale P, Zuehlsdorff S, Carr J, Li D., Carotid arterial wall MRI at 3T using 3D variable-flip-angle turbo spin-echo (TSE) with flow-sensitive dephasing (FSD)., J Magn Reson Imaging. 2010 Mar;31(3):645-54.
[3] Xie Y, Yang Q, Xie G, Pang J, Fan Z, Li D., Improved black-blood imaging using DANTE-SPACE for simultaneous carotid and intracranial vessel wall evaluation. Magn Reson Med. 2016 Jun;75(6):2286-94.
[4] Zhou Z, Li R, Zhao X, He L, Wang X, Wang J, Balu N, Yuan C., Evaluation of 3D multi-contrast joint intra- and extracranial vessel wall cardiovascular magnetic resonance., J Cardiovasc Magn Reson. 2015 May 27;17:41.
[5] Chiu B, Sun J, Zhao X, Wang J, Balu N, Chi J, Xu J, Yuan C, Kerwin WS., Fast plaque burden assessment of the femoral artery using 3D black-blood MRI and automated segmentation, Med Phys. 2011 Oct;38(10):5370-84. doi: 10.1118/1.3633899.
[6] Xie G, Zhang N, Xie Y, Nguyen C, Deng Z, Bi X, Fan Z, Liu X, Li D, Fan Z, DANTE-prepared three-dimensional FLASH: A fast isotropic-resolution MR approach to morphological evaluation of the peripheral arterial wall at 3 Tesla, J Magn Reson Imaging. 2016 Feb;43(2):343-51. doi: 10.1002/jmri.24986.
[7] Zhou Z, Chen S, Wu J, Zhao X, Börnert P, Yuan C, Deep Convolutional Neural Network Enhanced 3D High Resolution Turbo Spin Echo Intracranial Vessel Wall Imaging, ISMRM 2018.
[8] Eggers H, Brendel B, Duijndam A, Herigault G., Dual-echo Dixon imaging with flexible choice of echo times, Magn Reson Med. 2011 Jan;65(1):96-107.
Figures