2121
Effects of calcification on blood-flow pulsatility variation along the internal carotid artery in small vessel disease patients, a 7T MRI study
Rick J van Tuijl1, Ynte M Ruigrok2, Irene C van der Schaaf1, Gabriël J. E. Rinkel2, Birgitta K Velthuis1, and Jaco J.M. Zwanenburg1
1Radiology, UMC Utrecht, Utrecht, Netherlands, 2Neurology and Neurosurgery, Rudolf Magnus Institute of Neuroscience, UMC Utrecht, Utrecht, Netherlands
1Radiology, UMC Utrecht, Utrecht, Netherlands, 2Neurology and Neurosurgery, Rudolf Magnus Institute of Neuroscience, UMC Utrecht, Utrecht, Netherlands
Synopsis
The influence of intracranial internal carotid artery calcification (iICAC) over the whole internal carotid artery (ICA) trajectory was studied using 4D phase-contrast flow measurements (17 patients with cerebral small vessel disease (CSVD) and 17 healthy controls; 7T MRI). CT images of CSVD patients were used to calculate the iICAC from C3-C7 segments. Results showed a positive correlation between the presence and volume of iICAC with vPI in the CSVD group. iICAC contributes to an increase in vPI over the ICA from the extracranial C1 segment to the C7 segment just proximal to the circle of Willis compared to HC.
Introduction
Intracranial internal carotid artery calcification (iICAC) is predominantly present around the internal elastic lamina and (1) may thus increase the intracranial arterial stiffness’s in patients with and without cerebrovascular diseases. Increased stiffness leads to higher pulsatility in the smaller arteries, which could induce damage to the microcirculation that contributes to the development of cerebral small vessel disease (CSVD). However, a direct relationship between iICAC and velocity pulsatility in CSVD warrants further research. This feasibility study aims to assess the blood-flow pulsatility in patients with CSVD and matched healthy controls (HC), and to study the influence of iICAC on the reduction in pulsatility along the ICA towards the circle of Willis.Methods
Four-dimensional phase-contrast MRI (4D PC-MRI) acquisitions were used to acquire time-resolved measurements of blood velocities and volumetric flow rates simultaneously over the whole ICA trajectory. 4D PC-MRI parameters: resolution=0.8x0.8x0.8mm3, Venc=100cm/s, angulated transverse FOV=250x(anterior-posterior)x190(right-left) x20(feet-head)mm3, flip angle=15°. The acquisition time was approximately 4:55 min:sec for a heart rate of 60 bpm. We assessed blood-flow velocity in 17 patients with CSVD and 17 HC using 7T MRI, who were previously described (2) in a study focused on perforator artery pulsatility; see Table 1 for characteristics of the participants. The blood-flow velocity pulsatility index (vPI: (Vmax-Vmin)/Vmean) was calculated for all ICA segments (C1-C7). CT images of CSVD patients were used to calculate the iICAC from C3-C7; calcification could not be scored for C1 and C2 segments. An independent samples t-test was used to test for differences in vPI between the CSVD group and HC group for both right and left ICA at all locations. Influence of calcification scores on vPI were tested using a multivariable linear regression model with vPI as the dependent variable. To assess the influence of calcification, hypertension, age and sex on vPI per segment, we used a linear mixed-effects model since intra-subject values at the multiple segments are linked. The significant threshold was set at p<0.05.Results
Pulsatility variation along the ICA The CSVD group had significantly more hypertension and hypercholesterolemia compared to the HC (Table 1). Figure 1 shows the velocity pulsatility variation along the ICA for both CSVD and HC. In both groups, vPI increased along the ICA from C1 (extracranial segment) up to C4 (just past the bony carotid canal) and then decreased towards C7 (just proximal to the circle of Willis). This trend was consistent for both sides, in both groups (Table 2). The higher pulsatility seen in the CSVD group relative to the HC group was significant for all segments, except for the extracranial segment C1 (Table 2, Figure 2). The reduction in vPI over the carotid siphon diminished with increasing age in the CSVD group (p=0.044), but not in the HC group (p=0.121). The change in vPI between C1 and C7 was on average (left+right ICA) -4.6±3.6% in HC and +6.5±3.1% in CSVD. Relation pulsatility and calcification The presence of calcification correlated positively with vPI in both right (p=0.004) and left ICA (p=0.001) in the CSVD group. The combined left and right ICA volume of calcification correlated with the mean (left+right) ICA vPI (p<0.0001). The change in vPI between C1 and C7 was influenced by calcification volume (p=0.02), age (p=0.03), hypertension (p=0.072), but not by sex (p=0.433) (i.e. higher vPI at C7 with calcification, increasing age, and hypertension).Discussion
We hypothesize that the increased velocity pulsatility around the carotid canal that is seen in both patients and healthy controls is partly due to restricted distensibility of the ICA in this bony canal. Significantly increased pulsatility in the CSVD group for the C3 to C7 segments along the ICA can be partly explained by the presence of calcification resulting in increased vascular stiffness. This confirms a previous study performing transcranial Doppler ultrasonography in the cerebral vasculature in stroke patients (3). Arterial stiffening hinders the ability of arteries to dampen pulse pressures (4-6), leading to higher vPI along the ICA and subsequently in the cerebral microvasculature. Further research should include bigger CSVD populations to visualize flow hemodynamic differences in the ischemic stroke and intracerebral hemorrhage groups and gain more insight into the effect of iICAC in cerebrovascular disease and risk of reoccurrence due to increased vPI.Conclusion
A positive correlation was found between the presence and volume of intracranial ICA calcification with vPI in the CSVD group. Intracranial ICA calcification contributes to an increase (rather than attenuation) in vPI over the ICA from the extracranial C1 segment to the C7 segment just proximal to the circle of Willis compared to healthy controls.Acknowledgements
No acknowledgement found.References
1. Vos A, Van Hecke W, Spliet WG, Goldschmeding R, Isgum I, Kockelkoren R, et al. Predominance of Nonatherosclerotic Internal Elastic Lamina Calcification in the Intracranial Internal Carotid Artery. Stroke. 2016;47:221-3.2. Geurts LJ, Zwanenburg JJM, Klijn CJM, Luijten PR, Biessels GJ. Higher Pulsatility in Cerebral Perforating Arteries in Patients With Small Vessel Disease Related Stroke, a 7T MRI Study. Stroke. 2018:STROKEAHA118022516.
3. Park KY, Kim YB, Moon HS, Suh BC, Chung PW. Association between cerebral arterial calcification and brachial-ankle pulse wave velocity in patients with acute ischemic stroke. European neurology. 2009;61:364-70.
4. Humphrey JD, Na S. Elastodynamics and arterial wall stress. Annals of biomedical engineering. 2002;30:509-23.
5. Gaballa MA, Jacob CT, Raya TE, Liu J, Simon B, Goldman S. Large artery remodeling during aging: biaxial passive and active stiffness. Hypertension. 1998;32:437-43.
6. O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1-13.
7. Bouthillier A, van Loveren HR, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996;38:425-32.
Figures
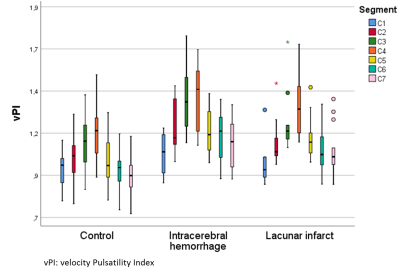
Figure 1: vPI
values for Healthy Control group, intracerebral hemorrhage and lacunar infarct
(combined CSVD group) per segment.
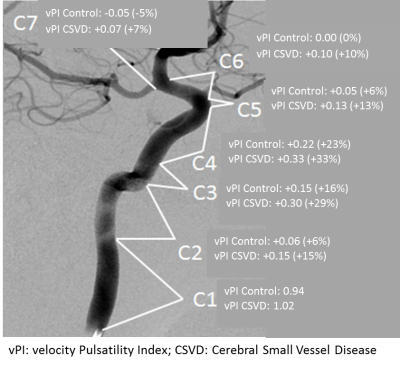
Figure 2: Visualization
of the vPI variation along the ICA segments for both groups using the
classification of Bouthillier for the Internal Carotid Artery segments (7).
The vPI values are averaged for the left and right ICA and represent the mean
values over the whole population. The change in vPI along the segments is shown
as delta (and percentage) with respect to the value at the C1 segment.
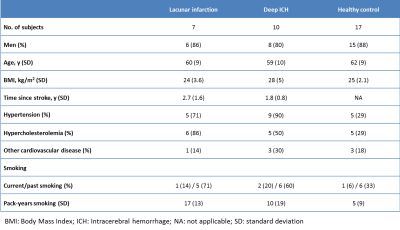
Table 1:
Baseline characteristics for the patients with lacunar infarction, deep
intracerebral hemorrhage and healthy controls.
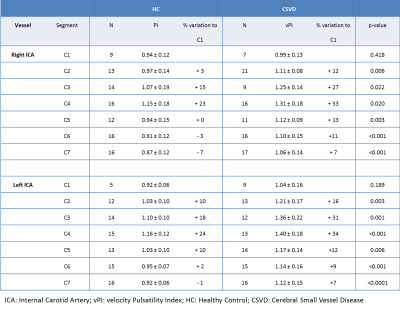
Table 2: Velocity
pulsatility index (vPI) values (mean ± SD) per segment of the ICA. N = number
of vPI measurements per vessel and segment, percentage difference is calculated
with respect to the value at the C1 segment.