2119
4D Flow Analysis of Left Atrium and Appendage in Patients with Atrial Fibrillation1Stephenson Cardiac Imaging Center, Libin Cardiovascular Institute of Alberta, Calgary, AB, Canada, 2Department of Medicine, University of Calgary, Calgary, AB, Canada, 3Diagnostic Imaging, University of Calgary, Calgary, AB, Canada, 4Department of Cardiac Science, University of Calgary, Calgary, AB, Canada
Synopsis
This study may be of interest for clinicians and researchers who study left atrial arrhythmias. This study demonstrated that left atrial stasis in the appendage is elevated in atrial fibrillation patients.
Intoduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia. The associated morbidity and mortality make AF a major socioeconomic burden. It occurs when electrophysiological or structural abnormalities alter atrial tissue to promote abnormal impulse formation. It causes insufficient contraction and hyper-coagulability in the LA that leads to thrombi formation mainly within LAA, hence the importance of flow evaluation plays a role. The risk of stroke increases about 5-folds in patients with AF and the CHA2DS2-VASc scores are used for the assessment of stroke risk.Aim
The study was sought to investigate whether left atrial (LA) functional measures correlate with CHA2DS2-VASc Score in predicting the increase risk of stroke.Methods and Materials
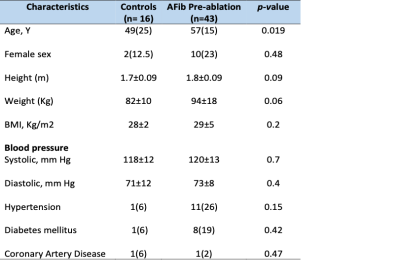
Cardiac MRI with 4D flow was performed in 59 subjects divided into 2 groups: healthy controls group (16 individuals) and AF group (43 patients in sinus rhythm prior to pulmonary veins catheter ablation procedure and with no cardiovascular disease). CHA2DS2-VASc risk score was calculated in all patients in accordance with current guidelines for the management of patients with AF. Each patient had a detailed medical history prior to MRI examination to document all clinical risk factors for stroke or thrombo-embolism.As per CHA2DS2-VASc scoring, patients were given one point for C: congestive heart failure or left ventricular systolic dysfunction, H: hypertension, D: diabetes mellitus, V:vascular disease, A: age 65-74, Sc: female gender, and two points for A₂: age ≥75 and S₂: prior stroke/transient ischemic attack or know thrombo-embolism.
CMR imaging examinations were performed on 3T MRI scanners (Skyra and Prisma, Siemens, Germany) with standardized cardiac MRI protocol and ECG-gated 4D flow with adaptive navigator respiratory gating with whole heart coverage. 4D flow imaging parameters were: Venc= 1.5-2.0 m/s, TE= 2.61-3.14 ms, TR= 4.9-5.9 ms, FOV= 200-420 mm x 248-368 mm, spatial resolution = 1.9-3.5x2.0-3.2x1.8-3.5 mm3, temporal resolution = 39-47 ms, and FA = 16°. 4D flow dataset pre-processing included: eddy-current correction, flow aliasing, and calculation of 3D phase contrast angiography (3D PC-MRA).
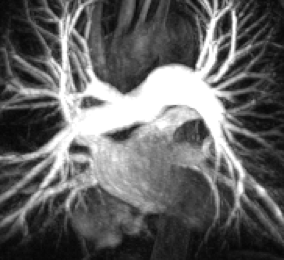
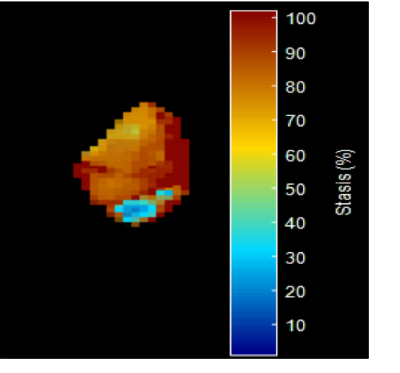
CMR parameters were used for cardiac function evaluation.Measurement of the left atrium was performed in the end-diastolic phase of the cardiac cycle using transverse 4 chamber and longitudinal 2-chamber views (figure 1). 3D PC MR angiogram (MRA) was generated for each subject using the pre-processed 4D flow MRI data and was used to manually perform a 3D segmentation (Matlab, Mathworks,MA) of the LA chamber and LAA (Figure 2). Atrial Velocity The 3D PC-MRA data were derived from the 4D flow data and used to quantify LA volume and to isolate the velocity data in the LA volume for all atrial voxels and all cardiac time frames. The resulting 4D flow MRI data provide information on 3-directional flow velocities [vx(t), vy(t), vz(t)] over the cardiac cycle within the segmented LA volume (t indicates time in the cardiac cycle). For further hemodynamic analysis, absolute atrial velocities V_mag=√(V_x^2+V_y^2+V_z^2 ) )3 4. Atrial stasis maps were derived as follows: for each voxel inside the segmented LA/LAA, the relative amount of flow stasis r stasis (% of absolute LA or LAA velocities <0.1 m/s) was calculated and normalized by the total number (NTot) of cardiac time frames: rstasis=nstasis/NTot×100. The threshold of 0.1 m/s was based on a previously conducted sensitivity analysis.
Statistical analyses were performed using SPSS. Normality test was performed using Shapiro-Wilk test to compare parameters between groups. The 2 groups were compared using 2-sample T-test or Mann-Whitney U test for quantitative variables and Chi-square test or Fisher’s exact test for qualitative variables. A p-value < 0.05 was considered significant.
Results
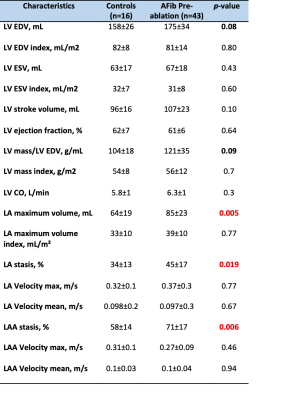
Baseline patient characteristics of the study population is summarized in Table 1. Changes in CMR function and geometric parameters are summarized in Table 2. Left ventricle ejection fraction, stroke volume, ESV and EDV were within normal limits for all patients, no heart failure cases. However, left atrial volume was abnormal (p-value 0.005), LA volume index was greater than the upper limit of normal (defined as mean +/- 2 SD) of 53 ml/m2 in controls in 3 patients (7%) . CHA2DS2-VASc score in pre-ablation patients (n=43 covering a range from 0 to 3. In AF patients left atrium and left atrial appendage stasis were significant (p-value 0.019 and 0.006 respectively). An example is demonstrated in Figure 3. Kruskal-Wallis test was used to determine the correlation of LA/LAA stasis with CHA2DS2-VASc score in pre-ablation patirents (n=43). The score were as follows 0, 1, 2, and 3 in 20 (47%), 16 (37%), 3 (7%), and 4 patients (9%), respectively. P-Value was significant p=0.02 in LA velocity mean and LAA stasis. (A value of P < 0.05 was considered statistically significant).Summary
This study demonstrated a significant increase in LA/LAA stasis in AF patients which may indicate the presence of a pressure overload in the LA. Elevated LA/LAA stasis may consequent LA thrombosis in AF patients. Future studies are needed to evaluate the impact of reported hemodynamic alterations of left atrial diseases on cardiac function.Acknowledgements
No acknowledgement found.References
1. Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Heuzey J Le, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Aha ACC, Force T, Smith SC, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Committee ESC, Practice FOR, Priori SG, Blanc J, Budaj A, Camm AJ, Dean V, Deckers JW, Morais J, Osterspey A, Zamorano JL. Circulation 2006;114;257-354. Online. 2007;
2. McGrath ER, Kapral MK, Fang J, Eikelboom JW, O’Conghaile A, Canavan M, O’Donnell MJ. Association of atrial fibrillation with mortality and disability after ischemic stroke. Neurology. 2013;81:825–832.
3. Markl M, Lee DC, Furiasse N, Carr M, Foucar C, Ng J, Carr J, Goldberger JJ. Left Atrial and Left Atrial Appendage 4D Blood Flow Dynamics in Atrial Fibrillation. Circ Cardiovasc Imaging. 2016;9:1–9.
4. Markl M, Lee DC, Ng J, Carr M, Carr J, Goldberger JJ. Left Atrial 4-Dimensional Flow Magnetic Resonance Imaging: Stasis and Velocity Mapping in Patients With Atrial Fibrillation. Invest Radiol. 2016;51:147–54.
5. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary. Circulation [Internet]. 2014;130:2071–2104. Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000040
6. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–72.
7. Lip GY, Nieuwlaat R, Pisters R, Lane DA CHR clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. C 2010 F 1;137(2):263-72.
Figures
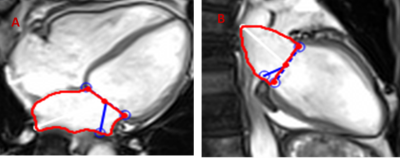
Figure1. Left Atrial (LA) Volume measurements in cardiac MRI in transverse 4-chamber view. B. In longitudinal 2-chamber view.