2105
Impact of Intraventricular Vortex on Kinetic Energy in Patients with Fontan Circulation
Xue-Zhe Lu1, Ming-Ting Wu2, Ken-Pen Weng3,4, and Hsu-Hsia Peng1
1Department of Biomedical Engineering and Environmental Sciences, National Tsing Hua University, Hsinchu, Taiwan, 2Department of Radiology, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan, 3Department of Pediatrics, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan, 4Department of Pediatrics, National Yang-Ming University, Taipei, Taiwan
1Department of Biomedical Engineering and Environmental Sciences, National Tsing Hua University, Hsinchu, Taiwan, 2Department of Radiology, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan, 3Department of Pediatrics, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan, 4Department of Pediatrics, National Yang-Ming University, Taipei, Taiwan
Synopsis
For patients with Fontan circulation, the intraventricular hemodynamics such as kinetic energy and vorticity might could be helpful with evaluating the cardiac function and the risks of negative long-term outcome. To investigate the impact of intraventricular vortex on kinetic energy in the of Fontan patients, we employed 4D flow magnetic resonance and evaluate intraventricular kinetic energy and vorticity in Fontan patients and found prolonged peaks in systolic kinetic energy and increased mean systolic vorticity. The strong correlation between kinetic energy and vorticity in systole showed a potential impact of vortex on kinetic energy during systole.
Introduction
The Fontan circulation is a palliative procedure which can save patients with single functional ventricle1. However, risks of ventricular dysfunction and heart failure elevated overtime after surgery2,3. Intraventricular kinetic energy (KE) provide a new insight into cardiac function in a variety of cardiovascular pathology4-6. A previous study reported young Fontan patients showed decreased KE in diastolic phase7. The vorticity (Vort) was proposed to characterize vortex flow in ischaemic heart disease patients and investigate the formation of vortex ring in diastolic phases in Fontan patients8,9. Even with normal mass and volume, the reduced systolic function was reported in patients late after Fontan operation10. Nevertheless, the intraventricular KE and Vort in Fontan patients during systole have not been thoroughly discussed even. The purpose of this study was to investigate the impact of intraventricular vortex on KE in the single functional ventricle of patients with Fontan circulation by 4D flow MRI.Methods
The study cohort recruited 15 Fontan patients (age:16.8±3.8 y/o, 9 males, 6 females) and 15 normal volunteers (age:21.4±0.8 y/o, 7 males, 8 females). The 4D flow data was acquired in a 3 Tesla MR scanner (Skyra, Siemens, Erlangen, Germany) with prospective ECG trigging (sampling 90% of cardiac cycle) and navigator-guided free-breathing technique. The scanning parameters were TR/TE=5.5/2.9 ms, voxel size=1.75×1.05×3.5 mm3, flip angle=15∘, and Venc=150 cm/s. Noise-masking, anti-aliasing, and eddy-current correction were applied to 4D flow data. Regions of intraventricle were outlined manually on cine steady-state free precession images in a short-axial view and applied to 4D flow data. The voxel-wise KE was calculated by:$$ {KE} = \frac{1}{2} V {\rho} {\vec{v}^{2}}$$where V is the voxel volume, ρ is the density of blood in the value of 1.05 g/ml 11, and $$$ \vec{v}$$$ represents flow velocity. The KE of the whole left ventricle was computed by summing up the KE of each intraventricular voxel and then normalized by stroke volume (SV) to produce the index of KEi:$${KE}_{i} = \frac{1}{SV} \sum KE$$The voxel-wise vorticity was computed by the curl of velocity. To sum up the vorticity of the whole LV, the magnitude of vorticity was multiplied with the voxel volume12 and indexed by SV as shown in equation:$$ {Vort}_{i} = \frac{1}{SV} \sum \mid \vec{\omega} \mid V$$The cardiac phase was normalized as percentage of end-systole (%ES). Mann-Whitney U test and Spearman's rho correlation coefficient were employed when appropriate. p < 0.05 was considered as statistical significance.Results
Table 1 lists demographics and cardiac MRI volumetric parameters in normal volunteers and Fontan patients. Compared to volunteers, Fontan patients exhibited dilated systolic and diastolic ventricular volumes, hypertrophic ventricular mass (all p<0.01), and reduced ventricular ejection function (52.3±8.4% vs 70.1±5.0%, p<0.001). Figure 1 displays the peak systolic intraventricular KE and vorticity of one representative Fontan patient and normal volunteer. The KE were higher near the outflow track. The vorticity were higher in Fontan patient compared to normal volunteer. As shown in Figures 2 and 3, the Fontan group presented similar peak systolic KEi and mean KEi compared to normal group, while the time-to-peak (TTPKE) of Fontan group was prolonged (52.5 ± 7.4 %ES vs. 44.9 ± 4.4 %ES, p=0.003). The significant increased systolic Vorti was exhibited in Fontan group (66.4 ± 20.9 s-1 vs 52.7 ± 10.9 s-1, p=0.032). Figure 4 illustrates that the mean systolic Vorti strongly correlated with peak and mean systolic KEi only in Fontan group (rho=0.83 and 0.84, respectively, both p<0.001).Discussion and Conclusions
In this study, Fontan patients exhibited prolonged systolic TTPKE and increased mean systolic Vorti. Consistent with previous study, Fontan group was with preserved peak and mean systolic KE13. Therefore, the prolonged systolic TTPKE in Fontan patients can be attributed to the impaired cardiac function, revealed by the reduced ventricular ejection fraction. The higher mean systolic Vorti in Fontan patients reflected the disturbed and turbulent intraventricular flow in the single functional ventricle. Patients with heart failure exhibited high systolic KE14 and increased vortices area during early systole15. In our study, Fontan patients were in a relatively early disease stage with preserved KEi. The preserved KEi and elevated Vorti illustrated that Vorti is a more sensitive and an early index to reveal the altered intraventricular flow. The strong positive correlation between mean Vorti and systolic KEi indicated that the Vorti may have adverse impact on the ventricular KE with disease progress. In conclusion, to realize the adverse impacts of intraventricular vortex on KE in the single functional ventricle of Fontan patients can be helpful to comprehend the intraventricular flow changes with disease progress.Acknowledgements
No acknowledgement found.References
- Fontan F, and Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26(3):240-248.
- Goldstein BH, et al. Relation of systemic venous return, pulmonary vascular resistance, and diastolic dysfunction to exercise capacity in patients with single ventricle receiving Fontan palliation. The American Journal of Cardiology. 2010;105(8):1169-1175.
- Khairy P, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117(1):85-92.
- Wong J, et al. Exploring kinetic energy as a new marker of cardiac function in the single ventricle circulation. Journal of Applied Physiology. 2018;125(3):889-900.
- Eriksson J, et al. Four-dimensional blood flow-specific markers of LV dysfunction in dilated cardiomyopathy. European Heart Journal–Cardiovascular Imaging. 2012;14(5):417-424.
- Jeong D, et al. Ventricular kinetic energy may provide a novel noninvasive way to assess ventricular performance in patients with repaired tetralogy of Fallot. The Journal of thoracic and cardiovascular surgery. 2015;149(5):1339-1347.
- Sjöberg P, et al. Decreased diastolic ventricular kinetic energy in young patients with Fontan circulation demonstrated by four-dimensional cardiac magnetic resonance imaging. Pediatric cardiology. 2017;38(4):669-680.
- Chan BT, et al. Quantitative analysis of intraventricular flow-energetics and vortex in ischaemic hearts. Coronary artery disease. 2018;29(4):316-324.
- Kamphuis VP, et al. Biventricular vortex ring formation corresponds to regions of highest intraventricular viscous energy loss in a Fontan patient: analysis by 4D Flow MRI. The international journal of cardiovascular imaging. 2018;34(3):441-442.
- Eicken A, et al. Hearts late after fontan operation have normal mass, normal volume, and reduced systolic function: a magnetic resonance imaging study. Journal of the American College of Cardiology. 2003;42(6):1061-1065.
- Trudnowski, RJ, and Rico RC. Specific gravity of blood and plasma at 4 and 37 C. Clinical chemistry. 1974;20(5):615-616.
- Kamphuis VP, et al. Scan–rescan reproducibility of diastolic left ventricular kinetic energy, viscous energy loss and vorticity assessment using 4D flow MRI: analysis in healthy subjects. The international journal of cardiovascular imaging. 2018;34(6):905-920.
- Kamphuis VP, et al. Disproportionate intraventricular viscous energy loss in Fontan patients: analysis by 4D flow MRI. European Heart Journal-Cardiovascular Imaging. 2018;20(3):323-333.
- Kanski M, et al. Left ventricular fluid kinetic energy time curves in heart failure from cardiovascular magnetic resonance 4D flow data. Journal of Cardiovascular Magnetic Resonance. 2015;17(1):111.
- Zhang H, et al. The left ventricular intracavitary vortex during the isovolumic contraction period as detected by vector flow mapping. Echocardiography. 2012;29(5):579-587.
Figures
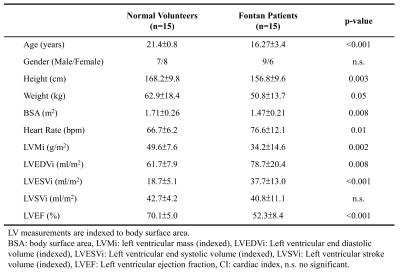
Table 1. Demographics
and CMR parameters in normal volunteers and Fontan patients.
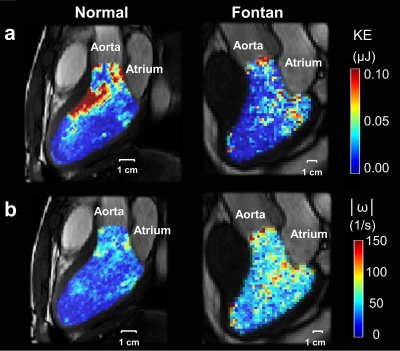
Figure 1.
The
intraventricular kinetic energy (a) and vorticity (b) maps of one 20-year-old
male with Fontan (left panel) and one 22-year-old male normal volunteer (right
panel) at peak systole.
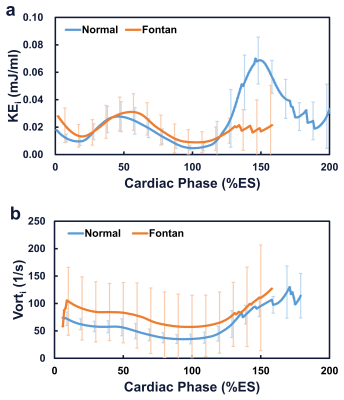
Figure 2.
The
time courses of indexed intraventricular kinetic energy (a) and vorticity (b)
of the whole left ventricle in normal and Fontan groups.
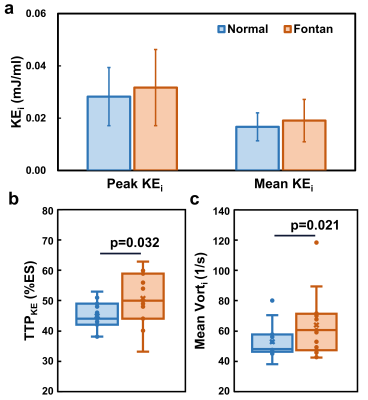
Figure 3.
(a)The
normal and Fontan groups presented similar peak and mean systolic indexed
kinetic energy (KEi). (b) The Fontan group displayed significantly higher
time-to-peak systolic KE (TTPKE) and (c) indexed systolic vorticity
(Vorti) than normal group.
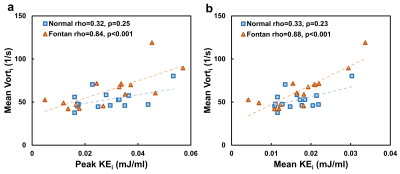
Figure 4. The mean systolic index vorticity (Vorti) exhibited significant
correlations with peak (a) and mean (b) systolic indexed kinetic energy (KEi)
only in Fontan group.