2097
Fourier Transform based Velocity-Selective Labeling Pulses for Myocardial ASL1Biomedical Engineering, University of Southern California, Los Angeles, CA, United States, 2Electrical Engineering, University of Southern California, Los Angeles, CA, United States, 3Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 4Radiology, Johns Hopkins University, Baltimore, MD, United States, 5F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
Synopsis
We present an improved velocity-selective (VS) labeling pulse for myocardial arterial spin labeling (ASL) that addresses limitations of prior pulses. The proposed pulse is designed using a Fourier Transform based Velocity-Selective (FT-VS) pulse train and optimized to label coronary blood while not labeling myocardium. Myocardial VSASL experiments were performed in healthy adult volunteers. The proposed pulse provided comparable measurements to FAIR ASL and a 2.5-fold reduction in physiological noise compared to a prior VS-ASL pulse.
Introduction
Myocardial arterial spin labeling (ASL) is a promising non-contrast technique for myocardial perfusion (MP) imaging which can be used to detect coronary artery disease1,2. Velocity-selective (VS) labeling was recently demonstrated for myocardial ASL3 to reduce sensitivity to transit delay, which can be a major source of error in perfusion quantification. The proposed VS labeling pulse had two major limitations which reduced the sensitivity of the myocardial VS-ASL; 1) A BIR8 saturation pulse was used which has half the labeling efficiency compared to inversion labeling used in FAIR and 2) VS-pulse design didn’t allow control over the velocity profile which increased the likelihood of spurious labeling of moving myocardium. Recently proposed Fourier Transform based velocity selective (FT-VS) pulses4-9 can be used to overcome the limitations of the myocardial VS-ASL method proposed by Jao et al3. In this study, we present a framework for optimization of the velocity profile of FT-VS pulses and use a carefully designed FT-VS pulse to demonstrate improved sensitivity for myocardial VS-ASL at 3T.Methods
Phantom and in-vivo experiments were performed on a 3T whole body scanner (Signa HDxT; General Electric Healthcare, Waukesha, WI) using an 8-channel cardiac coil receiver array. The human protocol was approved by our Internal Review Board and informed consent was obtained from all subjects.VS Pulse Design
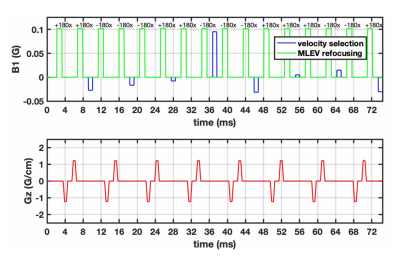
The proposed VS pulse (VS-prop) is based on designs by Shin et al and Qin et al5,7. The initial guess for the sub-pulse envelope was a linear-phase inversion with time-bandwidth product=6. π phase was added to alternate sub-pulse amplitudes to shift FOVv such that coronary velocities were inverted, and static tissues was unaffected. Sub-pulse amplitudes were optimized using Bloch simulations with minimum norm solver in MATLAB. Optimization was performed over b1 scale of 0.5-110 and off-resonance between ±125 Hz10,11, to maximize coronary ASL signal and minimize labeling of moving myocardium. Velocity ranges of 10-70 cm/s at rest, and 10-130 cm/s during stress were considered for coronary blood flow12-14,whereas a range of <±3 cm/s was used for myocardium15,16. These are the expected velocities during mid-diastole, when VS-labeling is performed. Silicon oil phantom (T1/T2 = 1111/227 ms)17,18 experiments were used to determine the gradient delays required to mitigate artifacts from gradient imperfections.
ASL experiments
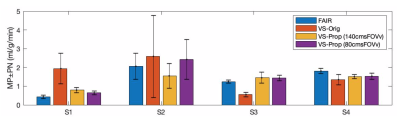
The performance of myocardial VS-ASL, with original and proposed labeling pulses, was compared to the reference FAIR-ASL method2 in four healthy volunteers (2M/2F, age 24-30Y). Previously published VS-ASL sequence with background suppression19 was used for myocardial VS-ASL and three velocity selective labeling pulses were used for comparison: 1) BIR8 VS-saturation (VS-Orig)19, 2) VS-Prop (FOVv = 140 cm/s), and 3) VS-Prop (FOVv = 80 cm/s) . Imaging was performed using balanced steady state free precession with previously published settings19.
Images were reconstructed using a custom implementation of GRAPPA20. Left ventricular myocardium was segmented in all images using semi-automatic segmentation, with previously published settings21. Global MP and PN were calculated for VS-ASL and FAIR as described by Jao et al.19 and Zun et al.2, respectively. For the VS-prop pulses calculation of MP was updated by; 1) dividing the signal (C-L) by 2 to account for inversion of flowing spins and 2) adjusting the T2-weighting term to reflect the duration of VS-Prop pulses.
Statistical equivalence of MP measurements between different methods was established using a two one-sided test (TOST) at a difference of 0.3 ml/g/min. Statistical difference in PN between methods was established using a paired T-test.
Results
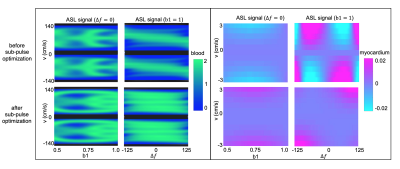
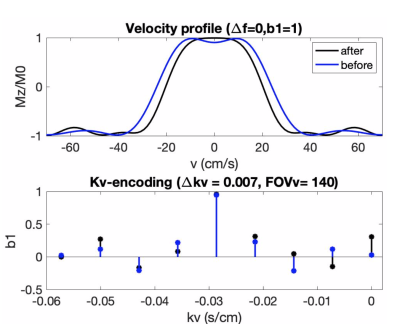
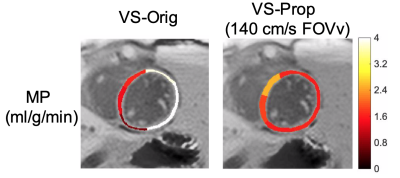
Figure 1 illustrates the proposed pulse (FOVv = 140 cm/s). Figure 2 illustrates reduced labeling of myocardium and improved labeling efficiency of blood after optimization. Figure 3 demonstrates the Fourier-relationship between b1 = 1 velocity profile and sub-pulse envelope. Figure 4 shows MP and PN measured using 4 ASL experiments (3 VS/1 FAIR) across 4 subjects. Figure 5 depicts a reduction in spurious labeling of moving myocardium in VS-Prop pulse compared to VS-Orig pulse.Discussion
This work demonstrates how FT-based velocity-selective RF pulses can be tailored for a specific application (myocardial ASL). Low-field applications and brain applications will likely require optimization over smaller range of off-resonance and b1 variations. Body applications with less motion will relax constraints on the stop-band of a tailored pulse. Other VS applications may benefit from consideration of T1 and T2 relaxation.Statistical testing is limited by analysis across only 4 subjects. MP measurements across methods presented in this study were physiologically reasonable but not statistically equivalent and statistical significance could be realized with inclusion of more datasets.
This study, like the previous study of myocardial VSASL (19), lacks experimental verification of insensitivity to transit delay. Theoretically, the proposed VS pulses are designed to label coronary blood and reduce transit delay effects. The extent to which transit delay is reduced is unknown. Future work is needed to experimentally verify insensitivity to transit delay.
Conclusion
We have demonstrated reduced physiological noise in myocardial VSASL with a specially tailored velocity-selective inversion pulse. This technique gives comparable MP measurements as FAIR ASL, making it a suitable candidate for multi-slice myocardial ASL and for myocardial ASL in patients with slow coronary flow.Acknowledgements
We thank Jia Guo and Eric Wong for helpful discussions. We gratefully acknowledge funding from the National Institutes of Health (R01-HL130494, PI: Nayak).References
1. Zun Z, Varadarajan P, Pai RG, Wong EC, Nayak KS. Arterial spin labeled CMR detects clinically relevant increase in myocardial blood flow with vasodilation. JACC. Cardiovasc. Imaging 2011;4:1253–61.
2. Zun Z, Wong EC, Nayak KS. Assessment of myocardial blood flow (MBF) in humans using arterial spin labeling (ASL): feasibility and noise analysis. MRM. 2009;62:975–983.
3. Jao TR, Nayak KS. Demonstration of velocity selective myocardial arterial spin labeling perfusion imaging in humans. MRM. 2018;80:272–278.
4. De Rochefort L, Maître X, Bittoun J, Durand E. Velocity-selective RF pulses in MRI. MRM. 2006;55:171–176.
5. Shin T, Hu BS, Nishimura DG. Off-resonance-robust velocity-selective magnetization preparation for non-contrast-enhanced peripheral MR angiography. MRM. 2013;70:1229–1240.
6. Levitt M. Broadband Heteronuclear Decoupling. JMR. 1981;47:328–330.
7. Qin Q, Shin T, Schar M, Guo H, Chen H, Qiao Y. Velocity-selective magnetization-prepared non-contrast-enhanced cerebral MR angiography at 3 Tesla: Improved immunity to B0/B1 inhomogeneity. MRM. 2016;75:1232–1241.
8. Shin T, Qin Q, Park JY, Crawford RS, Rajagopalan S. Identification and reduction of image artifacts in non–contrast-enhanced velocity-selective peripheral angiography at 3T. MRM. 2016;76:466–477.
9. Shin T, Qin Q. Characterization and suppression of stripe artifact in velocity-selective magnetization-prepared unenhanced MR angiography. MRM. 2018;80:1997–2005.
10. Sung K, Nayak KS. Measurement and characterization of RF nonuniformity over the heart at 3T using body coil transmission. JMRI. 2008;27:643–648.
11. Kellman P, Herzka DA, Arai AE, Hansen MS. Influence of Off-resonance in myocardial T1-mapping using SSFP based MOLLI method. JCMR. 2013;15:1–8.
12. Ofili EO, Labovitz AJ, Kern MJ. Coronary flow velocity dynamics in normal and diseased arteries. AJC. 1993;71.
13. Pereira VFA, De Carvalho Frimm C, Rodrigues ACT, Tsutsui JM, Cúri M, Mady C, Ramires JAF. Coronary flow velocity reserve in hypertensive patients with left ventricular systolic dysfunction. CC. 2002;25:95–102.
14. Anderson HV, Stokes MJ, Leon M, Abu-Halawa SA, Stuart Y, Kirkeeide RL. Coronary artery flow velocity is related to lumen area and regional left ventricular mass. Circulation 2000;102:48–54.
15. Gorcsan J, Gulati VK, Mandarino WA, Katz WE. Color-coded measures of myocardial velocity throughout the cardiac cycle by tissue Doppler imaging to quantify regional left ventricular function. AHJ. 1996;131:1203–1213.
16. Simpson R, Keegan J, Firmin D. Efficient and reproducible high resolution spiral myocardial phase velocity mapping of the entire cardiac cycle. JCMR. 2013;15:1–14.
17. Guo J, Meakin JA, Jezzard P, Wong EC. An optimized design to reduce eddy current sensitivity in velocity-selective arterial spin labeling using symmetric BIR-8 pulses. MRM. 2015;73:1085–1094.
18. Qin Q, van Zijl PCM. Velocity-selective-inversion prepared arterial spin labeling. MRM. 2016;76:1136–1148.
19. Jao TR, Nayak KS. Demonstration of velocity selective myocardial arterial spin labeling perfusion imaging in humans. MRM. 2017;00:1–7.
20. Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT - An eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. MRM. 2014;71:990–1001.
21. Javed A, Jao TR, Nayak KS. Motion correction facilitates the automation of cardiac ASL perfusion imaging. JCMR. 2015;17:51.
Figures