2095
Deep Learning for Radial Myocardial Perfusion Reconstruction using 3D residual booster U-Nets1Department of Biomedical Engineering, University of Utah, Salt Lake City, UT, United States, 2Department of Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 3Utah Center for Advanced Imaging Research (UCAIR), University of Utah, Salt Lake City, UT, United States, 4Department of Physics, University of Utah, Salt Lake City, UT, United States, 5Department of Cardiology, University of Utah, Salt Lake City, UT, United States
Synopsis
Although dynamic contrast enhanced (DCE) MRI has been successfully applied for characterizing coronary artery diseases, an acquisition scheme limited to 2-4 short axis slices restricts coverage of the left ventricle. Radial simultaneous multi-slice (SMS) has been shown to improve DCE cardiac perfusion by providing complete coverage of the left ventricle but also requires an increase in reconstruction time. Here we propose using a 3D residual booster U-Net to improve reconstruction time of spatio-temporal constrained reconstruction methods for radial SMS datasets. Results demonstrate promising improvements with a speed up in reconstruction by a factor of ~200.
Introduction
Dynamic contrast enhanced (DCE) MRI has been used to better differentiate between different soft tissue structures of the heart and has allowed for increased detection and characterization of different coronary artery disease states. DCE MRI of the heart is typically conducted through the acquisition of 2-4 short axis slices. However, this acquisition scheme provides incomplete coverage of the left ventricle of the heart and so provides limited information of the downstream effects of coronary artery disease states such as ischemia in the myocardium. Radial simultaneous multi-slice (SMS) has been shown to improve DCE cardiac perfusion by providing complete coverage of the left ventricle1,2. However, the increase in data undersampling from radial SMS also increases the reconstruction time required due to the use of advanced iterative reconstruction methods. To obtain high quality reconstructions with improved reconstruction time, we use a 3D residual booster U-Net to learn the iterative pixel tracking spatio-temporal constrained reconstruction (PT-STCR) with total variation constraints3. Improved image quality and reconstruction times are obtained in comparison to other network architectures.Methods
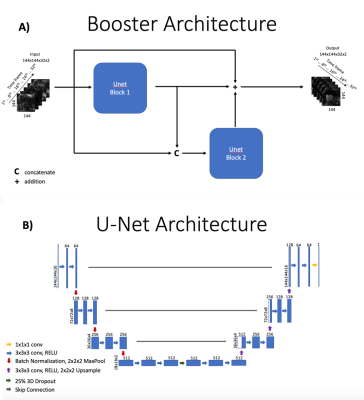
Data Acquisition and Processing: Gated radial SMS data were obtained from 10 subjects who were scanned at rest and/or stress. Multiple sets of radial SMS data were acquired with each cardiac cycle. Each SMS set sampled three parallel slices that were either short axis slices, two chamber long axis slices, or four chamber long axis slices3,4. Radial SMS data were processed to suppress streaking and principal component analysis (PCA) was used to compress the number of coils in the SMS data. SMS data were then interpolated onto Cartesian space using extended GROG5,6. Data from this step are reconstructed with the Inverse Fourier Transform and coil combined to create input to the networks. A preliminary STCR reconstruction with 30 iterations was conducted to create reference images from interpolated SMS data for respiratory state binning. A novel motion robust pixel tracking framework was employed with STCR (PT-STCR) to create fully reconstructed SMS images from reference images6. Images reconstructed from the PT-STCR are used as ground-truth images for training. All SMS data were acquired on Siemens 3T scanners. All radial data with golden ratio based spacing shared similar parameters as follows: TR = 2.7 ms, TE = 1.6 ms, FOV = 260 mm, ~1.8x1.8x8mm pixel size, 30 rays/frame, gadoteridol dose ~ 0.075 mmol/kg per injection and flip angle = 12.3D booster U-Net: Using the concept of boosting from machine learning7 and a residual learning framework8, a residual booster network using two 3D U-Nets9 was developed to learn PT-STCR images. Both 3D U-Nets contained ~40 million parameters. A weighted perceptual loss10 and mean absolute error loss was used for training. Ground truth images were obtained from the PT-STCR reconstruction pipeline described above. Input to the network consisted of 32 time frames of complex coil combined images reconstructed from undersampled k-t space data using the Inverse Fourier Transform. Training was done with data augmentation for 100 epochs on one NVIDIA P40 GPU and took ~27 hours. Training was performed on 10 datasets from 6 patients. Validation was performed on 2 datasets from 1 patient. Testing was performed on 6 datasets from 3 patients. Figure 1 demonstrates the structure of the booster network and the structure of the 3D U-Nets.
Network comparisons: In order to assess the effectiveness of the 3D residual booster U-Net architecture. Several other network architectures such as a 2D residual booster U-Net11, MoDL12, and CRNN13 are used for comparison. MoDL uses alternating CNN and data consistency blocks with weight sharing and conjugate gradient for improved MRI reconstruction. CRNN uses alternating RNN and data consistency blocks with hidden connections to incorporate temporal dynamics and iterative dependencies of iterative reconstruction algorithms for dynamic MRI reconstruction.
Results
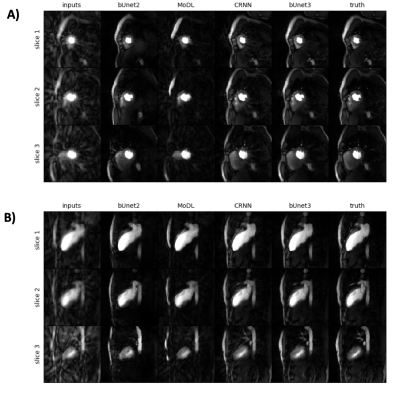
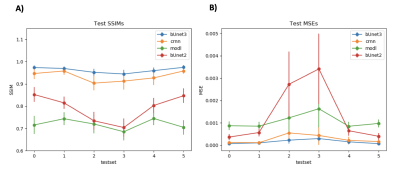
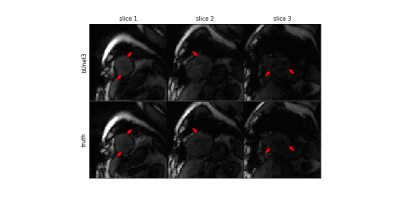
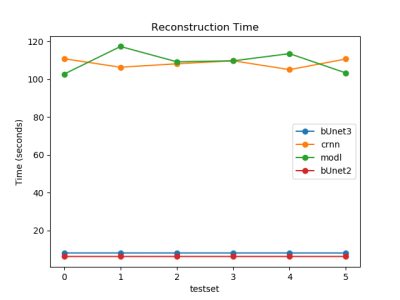
Figure 2 shows the results of the 3D residual booster U-Net on a gated radial SMS dataset not used in training in comparison to other deep learning architectures including a 2D booster U-Net , MoDL, and CRNN. Figure 3 demonstrates the SSIM and MSE values for each of the networks for all of the test sets. Figure 4 demonstrates the performance of the residual booster network on an ischemic test set. Figure 5 demonstrates the times required to reconstruct the test sets for each of the demonstrated networks.Discussion and Conclusions
Our results show that incorporating temporal dynamics is essential for accurately reconstructing gated radial SMS cardiac perfusion datasets. A 3D residual booster U-Net architecture demonstrates excellent reconstruction quality over other network architectures such as MoDL and CRNN while preserving ischemic myocardial regions of diseased test sets. This booster network also reconstructs these datasets significantly faster than the PT-STCR reconstruction (~25 minutes) as well as MoDL (~109 seconds) and CRNN (~109 seconds).Acknowledgements
This research was supported by the National Heart Lung and Blood Institute of the National Institutes of Health under award number RO1HL138082.References
1. G. Adluru, J. Mendes, Y. Tian, B. Wilson, E. DiBella, Ungated myocardial perfusion imaging with complete left ventricular coverage using radial simultaneous multi-slice imaging. ISMRM 2017.
2. G. Adluru, C. McGann, P. Speier, E.G. Kholmovski, A. Shaaban, E.V. Dibella, Acquisition and reconstruction of undersampled radial data for myocardial perfusion magnetic resonance imaging. Journal of magnetic resonance imaging: JMRI, 29 (2009) 466-473.
3. Y. Tian, J. Mendes, A. Pedgaonkar, M. Ibrahim, L. Jensen, J. Schroeder, B. Wilsion, E. Dibella, G. Adluru, Feasibility of multiple-view myocardial perfusion MRI using radial simultaneous multi-slice acquisitions. PloS ONE 14(2): e0211738.
4. E.V. Dibella, J. Mendes, M. Ibrahim, Y. Tian, B. Wilson, G. Adluru, Multiple sets of simultaneous multi-slice (SMS) for improved short and long axis coverage of myocardial DC perfusion. ISMRM 2018.
5. Y. Tian, G. Adluru, J. Mendes, E.V. Dibella, Evaluation of extended GROG and Toeplitz pre-interpolation methods on radial simultaneous multi slice MRI. ISMRM 2018.
6. Y. Tian, A. Pedgaonkar, J. Mendes, M. Ibrahim, B. Wilson, E.V. Dibella, G. Adluru, Rapid Motion Compensation Reconstruction for Dynamic MRI using Pixel Tracking Temporal Total Variation Constraint. ISMRM 2017.
7. J. Liu, E. Gong, T. Christen, G. Zaharchuk, Contrast-free MRI Constract Enhancement with Deep Attention Generative Adversarial Network. ISMRM 2019.
8. K. He, X. Zhang, S. Ren, J. Sun, Deep Residual Learning for Image Recognition, IEEE 2016.
9. O. Cicek, A. Abdulkadir, S. Lienkamp, T. Brox, O. Ronneberger, 3D U-Net: Learning Dense Volumetric Segmentation from Sparse Annotation, MICCAI 2016.
10. J. Johnson, A. Alahi, L. Fei-Fei, Perceptual Losses for Real-Time Style Transfer and Super Resolution,
11. O. Ronneberger, P. Fischer, T. Brox, U-Net: Convolutional Networks for Biomedical Image Segmentation. MICCAI, (2015) 234-241. 2016.
12. H. Aggarwal, M. Mani, M. Jacob, MoDL: Model Based Deep Learning Architecture for Inverse Problems, IEEE Transactions on Medical Imaging, (2019) 394-405.
13. C. Qin, J. Schlemper, J. Caballero, A. Price, J. Hajnal, D. Ruueckert, Convolutional Recurrent Neural Networks for Dynamic MR Image Reconstruction, IEEE Transactions on Medical Imaging 2017.
Figures