2073
Macromolecular Proton Fraction Mapping Enables Endogenous Contrast Between Normal and Infarcted Myocardium.1Radiology, University of Washington, Seattle, WA, United States, 2Pathology, University of Washington, Seattle, WA, United States, 3Institute for Stem Cells and Regenerative Medicine, University of Washington, Seattle, WA, United States, 4Comparative Medicine, University of Washington, Seattle, WA, United States, 5Cardiology, University of Washington, Seattle, WA, United States
Synopsis
This pilot study shows the feasibility of fast MPF mapping in cardiac applications in the clinical magnetic field using a large-animal model. The results demonstrate that MPF maps present the effective source of the quantitative soft tissue endogenous contrast in sub-acute myocardial infarction and can be obtained with high spatial and temporal resolution.
Introduction
Magnetization-transfer (MT) MRI has been proven to have high sensitivity and specificity for collagen content in healthy myocardium 1,2 and in patients with end stage renal disease 3. However, empirical indexes proposed in those studies are based on the ratio of signal intensities describing percentage saturation imposed by an MT pulse that does not permit true quantitation in tissues and strongly depend on the sequence parameters. Macromolecular proton fraction (MPF) is a quantitative parameter determining the MT effect in tissues. High MPF specificity to collagen has been demonstrated in the quantitative assessment of hepatic fibrosis 4. The goal of the current work was to develop a non-invasive cardiac MPF mapping approach for characterization of the myocardial tissue composition and assess it in a large-animal model of myocardial infarction (MI).Methods
Animals. Two groups of Yucatan mini-pigs were studied, the normal healthy animals (n=5) and pigs subjected to (MI modeled by 90-minutes LAD balloon inflation followed by reperfusion (n=10). Imaging was performed in 2 to 4 weeks after surgery. All animals were sedated and mechanically ventilated during imaging study.Image Acquisition. Animals were imaged using a 3T Philips Ingenia whole body scanner. The imaging protocol included three breath-hold cardiac-gated two-dimensional (2D) scans with 0.49x0.49 mm2 resolution with single 8 mm slice and 10 cardiac phases: (1) 2D MT-prepared spoiled gradient echo (GRE) sequence with TR/TE=34/7 ms, excitation flip angle α=10°, slice thickness 8 mm; (2, 3) two spoiled-GRE sequences providing T1 and proton-density (PD) contrast weightings with TR/TE=20/7 ms and excitation flip angle α=25°and α=4°, respectively. Total scan time was 48 sec. To determine localization of myocardial scar in the infarcted pigs, late gadolinium enhanced (LGE) images were obtained 10 min after iv bolus administration of the MRI contrast agent ProHance (0.12 mM/kg, Bracco Diagnostics Inc.).
Image Reconstruction and Analysis. 2D MPF maps were reconstructed from three source images (MT-, PD-, and T1-weighted) using the single-point method with the synthetic reference 5 image using the custom-written software. T1 maps were calculated in the same 2D short axis slice of the heart from the two variable flip angle images. Reconstruction was performed with correction coefficients taking into account the slice profile effect, which were determined from Bloch equation simulations. The MPF and T1 values were measured from the corresponding maps in the regions of interest (ROIs) centered over the normal and infarcted areas of the myocardium.
Results
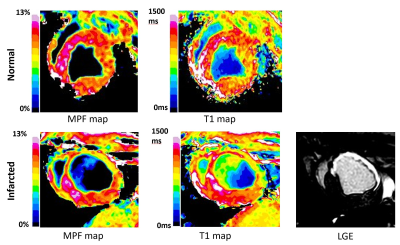
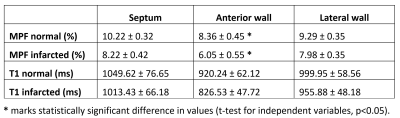
To enable MPF mapping of the pig heart in vivo, we have combined several technical solutions which allowed critical improvements in spatial resolution and time efficiency while maintaining the pulsed steady-state in combination with cardiac gating. MPF technique resulted in 10 maps over one cardiac cycle, which allowed dynamic CINE-type imaging of cardiac tissue composition. The infarcted area was clearly observable as a hypointense zone on MPF maps with the localization well corresponding to the enhancement area in LGE images (Figure 1). MPF maps also provided effective blood suppression (MPF of blood is 0) and were not affected by flow artifacts. The MPF and T1 values for healthy (lateral wall) and infarcted zones (septum, anterior wall) of the pig heart are listed in Table 1. The MPF values measured in the anterior wall of the heart were significantly smaller in the infarcted (6.05 ± 0.55 %) as compared to healthy (8.36 ± 0.45 %) animals (p<0.05). Unaffected myocardial tissue showed similar MPF in both animal groups. There was no significant difference between T1 values in the infarcted and normal myocardium.Conclusion
This is the first implementation of MPF mapping technique for cardiac applications. Cardiac MPF mapping showed a utility as a new approach for non-contrast imaging and quantitative characterization of myocardial infarction. MPF maps in the swine sub-acute myocardial infarction model showed higher sensitivity to post-infarction scar formation as compared to T1 maps. More research is needed to establish a pathological background of MPF reduction in the infarcted area that may be driven by other than collagen proliferation factors.Acknowledgements
No acknowledgement found.References
1. Lopez KN, Mukherjee R, Whitaker R, Razavi J, Muñoz R, Prieto C, Roujol S, Botnar R. Non-Contrast Free Breathing and Motion Corrected 3D Whole Heart Quantitative Magnetization Transfer Imaging for Assessment of Myocardial Fibrosis. ISMRM 2017 Proceedings. Abstract 0046.
2. Stromp TA, Leung SW, Andres KN, Jing L, Fornwalt BK, Charnigo RJ, Sorrell VL, Vandsburger MH. Gadolinium free cardiovascular magnetic resonance with 2-point Cine balanced steady state free precession. J Cardiovasc Magn Reson. 2015;17(1):90.
3. Stromp T, Spear TJ, Holtkamp RM, Andres KN, Kaine JC, Alghuraibawi WH, Leung SW, Fornwalt BK, Vandsburger MH. Quantitative Gadolinium-Free Cardiac Fibrosis Imaging in End Stage Renal Disease Patients Reveals A Longitudinal Correlation with Structural and Functional Decline. Sci Rep. 2018;8(1):16972.
4. Yarnykh VL, Tartaglione EV, Ioannou GN. Fast macromolecular proton fraction mapping of the human liver in vivo for quantitative assessment of hepatic fibrosis. NMR Biomed. 2015;28(12):1716-1725.
5. Yarnykh VL. Fast macromolecular proton fraction mapping from a single off-resonance magnetization transfer measurement. Magn Reson Med. 2012;68(1):166-178.