2072
High Resolution Imaging of the Ex-Vivo Porcine Heart at 7T Using a Dedicated Custom Built 8TX/16RX Array: Quality Assessment1Chair of Cellular and Molecular Imaging, Comprehensive Heart Failure Center (CHFC), University Hospital Würzburg, Würzburg, Germany, 2Chair Tissue Engineering and Regenerative Medicine (TERM), University Hospital Würzburg, Würzburg, Germany
Synopsis
MRI measurements of ex-vivo hearts at ultrahigh field strengths (≥7T) can provide high resolution, high fidelity ground truth data that complement clinical cardiac MRI. In this study we demonstrate ex-vivo sample preparation steps and capabilities of a custom built, multiple element transceiver array dedicated to high resolution imaging of ex-vivo hearts. Measurements included whole heart data for T2* maps, a high resolution 3D FLASH, as well as high resolution DTI. We found that receive sensitivity of the dedicated coil was superior to a commercial head coil and that SNR was similar, even without the application of B1-shim.
Introduction
Ultra-high field strengths (≥7T) have been shown to provide improved morphological1 and functional2,3 information in the brain leading to an increasing interest in cardiac applications as well. These improvements with respect to clinical 1.5 and 3T MRI are possible due to increased signal-to-noise and contrast-to-noise ratios, as well as due to other/enhanced contrast mechanisms. Increased field strength, however, demands better B0 and B1+ homogeneity. Thus, ex-vivo measurements become an important tool, in order to achieve ultrahigh resolution and high fidelity data. In this study we compare data acquisition with a custom built coil with optimized filling factor for ex-vivo pig hearts, and we compare results to those obtained with a commercially available head coilMethods
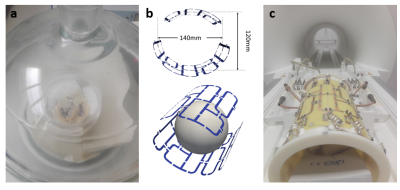
All hearts (n=3) were kindly provided following the animals approved use (55.2 2532-2-256, District Government of Lower Franconia, Germany) in another study. Following excision, atria of the hearts were removed in order to ease the release of trapped air. Fixation was achieved via immersion in 10% neutral buffered Formalin within 3 hours of cardiac arrest. Hearts were placed in a plastic container and the sample position fixed using Fomblin soaked sponges. The container was then slowly filled with Fomblin and excess air removed from the sponges and the heart using a vacuum desiccator as indicated in Figure 1a.Measurements were performed on a 7T whole-body MRI system (Siemens MAGNETOM™ Terra) using a 1Tx/32Rx head coil (sTx, diameter: ~20cm) and a custom built 8TX/16RX array (pTx, diameter: ~11cm, Figure 1b and c). Total measurement time for all sequences was ~9 hours. At this point, we did not apply any B1-shim.
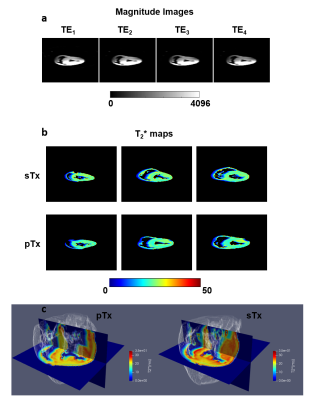
T2* maps with 1mm isotropic resolution were created based on 2D gradient multi-echo sequence data acquired with both coils for one heart. Measurement parameters were: number of averages: 32, TR: 800ms, nine echoes per excitation (TE): 2.5ms to 18.7ms.
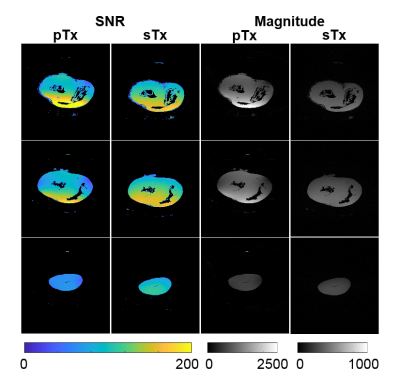
Ultrahigh resolution images for both coils were acquired using a 3D FLASH sequence with the following parameters: FOV: 160mm, FOVphase: 62.5%, 0.125 mm interpolated in-plane resolution, slice thickness: 0.5mm, bw: 300 Hz/Px, averages: 15. Noise for SNR calculation in each slice was determined using a 100x100 pixel ROI in the top left corner.
In addition, we used a spin echo diffusion sequence with Stejskal-Tanner diffusion encoding to acquire whole heart diffusion tensor images with isotropic resolution of 0.8mm and 1.6mm. Further parameters for these acquisitions were: TE = 56 / 73, acceleration factor R=3, 30 diffusion directions after Skare4 (b = 2000 s/mm2), averages: 50, bw = 1414 Hz/px.
All data processing was done using DSI Studio5 and Matlab (MathWorks, Natick, USA)
Results
The preparation setup for ex-vivo hearts is depicted in Figure 1. Regarding the efficiency or air removal, we found that consistent vacuum throughout a whole night was necessary to remove excess air, leading to completely soaked sponges and no signal besides that from cardiac tissue.Figure 2a shows representative magnitude images from the four shortest echo times during T2* measurements using the ex-vivo coil. Reducing the image scaling factor from 1 to 0.1 was necessary to avoid signal saturation in T2* measurements using this coil. No such saturation was observed using the head coil. Average values for T2* in the whole heart were 19.4±8.3ms and 18.6±7.7ms (Figure 2b and c) for the sTx and pTx coil, respectively.
Ultra-high resolution T1 weighted images are shown in figure 3. Mean SNR values throughout the whole volume ranged from 95-139 and 60-141 for sTx and pTx coils, respectively. While SNR in the measurements was similar between sTx and pTx images, we found that signal intensities were approximately three times higher using the pTx coil with optimized filling-factor.
Figure 4 shows representative high resolution tractography for one measurement using the ptx coil, visualizing distinct structures of the heart.
Discussion
Measured T2* values are in good agreement with previously reported T2* values for hearts at 7T using similar fixation duration6.The high signal in both T2* and the 3D FLASH sequence demonstrate the high sensitivity of the ex-vivo pTx coil, which was optimized for ex-vivo imaging of the pig heart. For both coils we observed higher signal intensities on the bottom side of the coil. For the ex-vivo coil this is due to the fact that the lower coil part hosts 10 array elements, while the top part hosts the remaining six lements. For the sTx coil this is most likely caused by the bigger distance of coil elements with regard to the top of the container and a sub-optimal filling-factor. Future assessment of coil sensitivity will be based on the vendor-provided coil utility SNR map and B1+-mapping.
With respect to machine and deep learning approaches, high fidelity ground truth data, becomes increasingly important. In this study we show a dedicated setup for sample preparation and custom built hardware, enabling the acquisition of such data.
Conclusion
The dedicated ex-vivo coil array demonstrates high efficiency with regard to receive properties, enabling anatomical and structural imaging with ultra-high resolutions. With future measurements regarding transmit and B1-shim capabilities we plan to optimize this setup for ultra-high resolution ex-vivo imaging of the heart.Acknowledgements
Financial support: German Ministry of Education and Research (BMBF, grant: 01E1O1504).References
1. Trattnig S, Bogner W, Gruber S, et al. Clinical applications at ultrahigh field (7 T). Where does it make the difference? NMR in biomedicine 2016;29(9):1316-34.
2. Goncalves NR, Ban H, Sánchez-Panchuelo RM, et al. 7 Tesla fMRI Reveals Systematic Functional Organization for Binocular Disparity in Dorsal Visual Cortex. The Journal of Neuroscience 2015;35(7):3056-72.
3. Bogner W, Chmelik M, Andronesi OC, et al. In vivo 31P spectroscopy by fully adiabatic extended image selected in vivo spectroscopy: A comparison between 3 T and 7 T. Magnetic resonance in medicine 2011;66(4):923-30.
4. Skare S, Hedehus M, Moseley ME, et al. Condition Number as a Measure of Noise Performance of Diffusion Tensor Data Acquisition Schemes with MRI. Journal of magnetic resonance. 2000;147(2):340-52.
5. Yeh F-C. DSI Studio. http://dsi-studio.labsolver.org. Accessed April 22, 2016.
6. Lohr D, Terekhov M, Kress S, et al. Ex-Vivo Diffusion Tensor Imaging of the Porcine Heart at Ultra High Field Strength - 7T: Impacts of Tissue Fixation Duration and Stimulated Echo Mixing Time. Proc. of the 26th Annual Meeting of the ISMRM 2019.
7. Terekhov M, Elabyad I, Lohr D, et al. In-house Built Optimized 8TX/16RX Array for Ultra-High Resolution Ex-Vivo Myocardial Tissue Characterization with 7T MRI: Initial Experience and Quality Assessment. Proc. of the 26th Annual Meeting of the ISMRM 2019.
Figures