2068
Respiratory motion-registered isotropic 3D whole-heart T2 mapping in patients with suspected inflammatory myocardial injury1Department of Noninvasive Cardiac Diagnostics, Medical University of Gdansk, Gdansk, Poland, 2Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 32nd Department of Radiology, Medical University of Gdansk, Gdansk, Poland, 4Siemens Healthineers, Erlangen, Germany, 5Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 6Center for Biomedical Imaging (CIBM), Lausanne, Switzerland
Synopsis
T2 mapping can be used to effectively detect myocardial edema and inflammation. However, the focal nature of myocardial inflammation may render 2D approaches suboptimal and make whole-heart isotropic 3D mapping desirable. Unfortunately, at 1.5T, self-navigated 3D radial bSSFP results in too noisy images for adequate T2 mapping. In this study, we therefore used a respiratory motion-resolved reconstruction together with image registration to improve the 3D T2 mapping precision and accuracy at 1.5T in patients with inflammatory myocardial injury. The resulting myocardial T2 values matched those of the routine 2D T2 maps, with no discernible bias and slightly lower precision.
Background
Myocardial inflammation can be detected as elevated T2 relaxation times on T2 maps [1]. However, the focal nature of acute non-ischemic inflammatory myocardial injury in a spectrum of inflammatory conditions may render 2D T2 mapping of the heart suboptimal due to either insufficient spatial coverage or prolonged scan times. Hence, a non-invasive whole-heart isotropic 3D T2 quantification [2] is desirable in this setting, as was demonstrated previously in patients with acute graft rejection at 3T [3]. 3D techniques with radial [2], Cartesian [4,5], or hybrid [6] trajectories can be chosen, with intrinsic advantages such as inherent motion robustness for radial imaging and high signal-to-noise for Cartesian imaging. Unfortunately, at 1.5T, self-navigated 3D radial bSSFP results in too noisy images for adequate myocardial T2 mapping. To address this shortcoming, in this study we aimed to use respiratory motion-resolved reconstruction together with image registration of the resulting 4D images to decrease noise and motion artifacts, and thus to improve 3D T2 mapping precision and accuracy at 1.5T. These respiratory motion-resolved T2 maps were then compared to self-navigated 3D maps that were reconstructed from the same data, as well as to routine 2D maps.Materials and Methods
This study was approved by the Institutional Review Board. Consecutive patients with suspected acute non-ischemic inflammatory myocardial injury (n=12, 6 cardiac sarcoidosis, 2 myocarditis, 2 acute graft rejection, 2 systemic sclerosis; 5 (42%) female, age 51±11y) were recruited. On a 1.5T clinical scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany), routine 2D T2-prepared bSSFP T2 maps (pixel size 1.9x1.9mm2, slice thickness 8mm) [7] were acquired in a short-axis (SAX) orientation. Next, three 3D radial bSSFP volumes were acquired during free breathing (isotropic voxel size 1.6mm3, T2prep=0,30,60ms, flip angle=35°, interleaves of 50 lines acquired every third heartbeat) [2,8] using a prototype sequence.For the respiratory motion-resolved reconstruction, a principal component analysis (PCA) was performed on the superior-inferior profiles that were acquired at the start of each interleave [9,10], and to partition the dataset into 4 different respiratory states. 4D (x-y-z-respiratory dimensions) images were then reconstructed with a compressed sensing algorithm that exploits sparsity along the respiratory dimension [11]. All respiratory bins were translationally and then non-rigidly registered to the end-expiration bin with Elastix [12], and were then averaged. After a second, similar, registration of the resulting three averaged T2-prepared images, pixel-wise T2 mapping [2] was performed. Since the motion is no longer resolved after these registrations, we named the resulting 3D map “motion-registered” T2 maps.
For the self-navigated reconstruction from the same 3D radial data, the 1D displacement of the left-ventricular blood pool along the superior-inferior readouts acquired at the start of each interleave was used to correct each interleave for respiratory motion in k-space prior to image reconstruction [8]. The resulting three 3D images were translationally and then non-rigidly registered, and pixel-wise T2 mapping [2] was performed.
The T2 values of the entire visible myocardium in the routine 2D maps and a matching single slice in both 3D volumes were then measured and compared using Matlab.
Results and Discussion
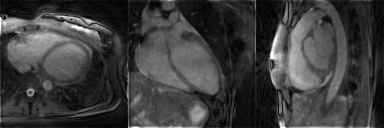
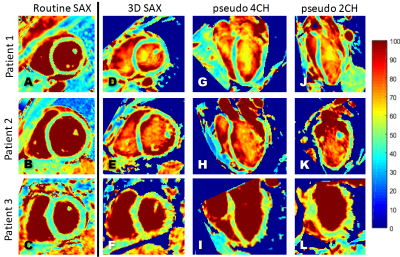
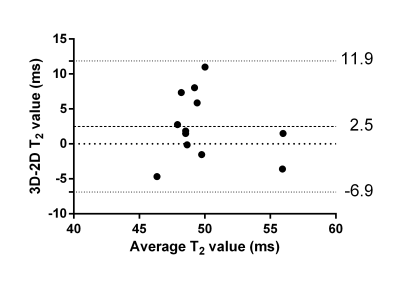
The respiratory motion-resolved reconstruction resulted in visibly well-separated motion states (Figure 1), and motion-registered isotropic 3D T2 maps of the heart were successfully obtained in all patients (Figure 2). Their myocardial T2 values matched those of the routine maps (51.1±3.5ms vs. 48.6±4.1ms, respectively, P=0.10), while the Bland-Altman analysis demonstrated that there was no discernible trend or bias when compared to the routine maps (Figure 3).The motion-registered 3D T2 mapping precision was slightly lower than that of the routine technique, as evidenced by the myocardial T2 standard deviation (5.7±1.3ms vs. 4.5±1.9ms, P=0.03). This is most likely a combination of several factors: on the one hand, the compressed sensing reconstruction removes some noise, while on the other hand, residual 3D radial undersampling artifacts and local misregistration artifacts, as well as the much smaller voxel size will increase the T2 standard deviation. As expected, the self-navigated version resulted in significantly higher myocardial T2 values (58.2±9.2ms, P<0.001 versus both other techniques) and T2 standard deviation (9.7±5.3ms, P<0.001 versus both other techniques) compared to the routine maps, confirming the need for the motion-resolved reconstruction.
Conclusions
Respiratory motion-resolved 3D radial imaging at 1.5T led to precise and accurate isotropic 3D whole-heart T2 maps in a small patient cohort. Future efforts will be directed towards studies in larger patient cohorts as well as accelerating the mapping.Acknowledgements
No acknowledgement found.References
1. Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, Taylor AJ, Thompson R, Ugander M, van Heeswijk RB, Friedrich MG. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson. 2018 Feb 7;20(1):9. doi: 10.1186/s12968-017-0408-9
2. van Heeswijk RB, Piccini D, Feliciano H, Hullin R, Schwitter J, Stuber M. Self-navigated isotropic three-dimensional cardiac T2 mapping. Magn Reson Med. 2015 Apr;73(4):1549-54. doi: 10.1002/mrm.25258
3. van Heeswijk RB, Piccini D, Tozzi P, Rotman S, Meyer P, Schwitter J, Stuber M, Hullin R. Three-Dimensional Self-Navigated T2 Mapping for the Detection of Acute Cellular Rejection After Orthotopic Heart Transplantation. Transplant Direct. 2017 Mar 28;3(4):e149. doi: 10.1097/TXD.0000000000000635
4. Ding H, Fernandez-de-Manuel L, Schär M, Schuleri KH, Halperin H, He L, Zviman MM, Beinart R, Herzka DA. Three-dimensional whole-heart T2 mapping at 3T. Magn Reson Med. 2015 Sep;74(3):803-16. doi: 10.1002/mrm.25458
5. Milotta G, Ginami G, Bustin A, Neji R, Prieto C, Botnar RM. 3D Whole-heart free-breathing qBOOST-T2 mapping. Magn Reson Med. 2019 Oct 21. doi: 10.1002/mrm.28039. [Epub ahead of print] 6. Yang HJ, Sharif B, Pang J, Kali A, Bi X, Cokic I, Li D, Dharmakumar R. Free-breathing, motion-corrected, highly efficient whole heart T2 mapping at 3T with hybrid radial-cartesian trajectory. Magn Reson Med. 2016 Jan;75(1):126-36. doi: 10.1002/mrm.25576. Epub 2015 Mar 6.
7. Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009 Dec 30;11:56. doi: 10.1186/1532-429X-11-56
8. Piccini D, Littmann A, Nielles-Vallespin S, Zenge MO. Epub 2011 Dec 28. Respiratory self-navigation for whole-heart bright-blood coronary MRI: methods for robust isolation and automatic segmentation of the blood pool. Magn Reson Med. 2012 Aug;68(2):571-9. doi: 10.1002/mrm.23247.
9. Di Sopra L, Piccini D, Coppo S, Stuber M, Yerly J. An automated approach to fully self-gated free-running cardiac and respiratory motion-resolved 5D whole-heart MRI. Magn Reson Med. 2019 Dec;82(6):2118-2132. doi: 10.1002/mrm.27898
10. Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med. 2016 Feb;75(2):775-88. doi: 10.1002/mrm.25665
11. Piccini D, Feng L, Bonanno G, Coppo S, Yerly J, Lim RP, Schwitter J, Sodickson DK, Otazo R, Stuber M. Four-dimensional respiratory motion-resolved whole heart coronary MR angiography. Magn Reson Med. 2017 Apr;77(4):1473-1484. doi: 10.1002/mrm.26221
12. Klein S, Staring M, Murphy K, Viergever MA, Pluim JP. Elastix: a toolbox for intensity based medical image registration. IEEE Trans Med Imaging. 2010 Jan;29(1):196-205. doi: 10.1109/TMI.2009.2035616
Figures