2067
Accelerated and non-rigid motion-corrected 3D T2 mapping in a 3T PET-MR system1Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
Simultaneous 18F-FDG PET-MR imaging has shown promise for improved diagnostic accuracy of inflammatory cardiac diseases, such as cardiac sarcoidosis. However, respiratory motion and mis-registration between free-breathing 3D PET and conventional 2D breath-held MR images remain a challenge that has hindered clinical adoption of this technique. Here we introduce a 3x-accelerated free-breathing motion-corrected 3D whole-heart T2-mapping prototype sequence, which provides characterisation of myocardial inflammation while additionally providing non-rigid respiratory deformation fields to correct simultaneously acquired PET data. Results from phantom, healthy subjects and patients show that the method produces good-quality high-resolution 3D T2 maps from an efficient scan of ~10 minutes.
Introduction
Simultaneous 18F-FDG PET-MR imaging has shown promise for improved diagnostic accuracy of inflammatory cardiac diseases, such as cardiac sarcoidosis.1-2 PET-MR imaging of cardiac sarcoidosis can provide information about myocardial function, the pattern of injury and disease activity from a single scan. However, fusion and interpretation of conventional cardiac MR data, usually acquired in 2D under multiple breath holds, and simultaneously acquired 3D free-breathing PET data remain a challenge due to misalignment and differences in geometry between both modalities. Moreover, respiratory motion degrades both image quality and quantification of PET and MR images, hindering the clinical adoption of this technique.Here we introduce an efficient free-breathing 3D whole-heart T2-mapping sequence, which provides myocardial inflammation characterisation and non-rigid respiratory motion fields to correct simultaneously acquired PET data in a 3T hybrid PET-MR system. This is achieved by extending a previously introduced approach for 3D translational motion-corrected T2 mapping at 1.5T.3 The proposed sequence potentially enables complementary and fully co-registered motion-compensated T2 mapping and 18F-FDG PET imaging for inflammatory characterisation of cardiac disease.
Methods
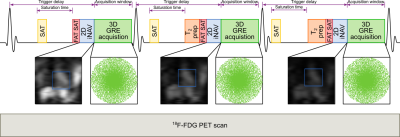
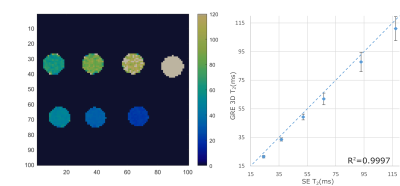
Acquisition & Reconstruction: A free-breathing 3D whole-heart T2-mapping prototype sequence (Figure 1) was implemented on a 3T PET-MR system (Biograph mMR, Siemens Healthcare, Erlangen, Germany). Three spoiled gradient echo datasets are acquired with an undersampled variable-density Cartesian trajectory4 with an acceleration factor of 3x and varying magnetisation preparation schemes. A saturation pulse (SAT) is acquired at the start of each heartbeat to reset the magnetisation and render the sequence heart-rate independent, followed by a fat saturation pulse and T2-preparation pulses of different durations (0, 28 and 55ms) immediately prior to the imaging sequence. Image navigators (iNAVs)5 are integrated in the sequence to enable 100% respiratory scan efficiency and predictable scan time. Acquired 3D data are assigned to different respiratory positions (bins) based on the foot-head (FH) motion estimated from the iNAVs. Images for each respiratory bin are reconstructed with iterative SENSE6 and intra-bin FH and right-left (RL) iNAV-based translational motion correction. 3D bin-to-bin non-rigid motion is estimated from the respiratory-resolved bin images and incorporated in a motion-compensated reconstruction framework7 with patch-based low-rank regularization (HD-PROST3) to produce motion-compensated datasets. T2-maps are computed using dictionary-matching9. The non-rigid motion fields estimated with this approach can be used to motion-correct both the MR and PET data to the same respiratory position8, enabling direct fusion of both datasets for analysis and interpretation.Imaging & Analysis: The accuracy of the proposed 3D T2 mapping was investigated in a phantom with different agarose concentrations (0.8, 1, 1.5, 2, 3 and 5%) with the same parameters as for the in-vivo scans (acquisition time = 3min43s), and compared to a gold-standard 2D multi-echo spin-echo sequence (TR=10s, TE=12, 28 and 55ms, matrix size = 128x128, resolution 2x2x8mm3, acquisition time = 21min10s for each TE).
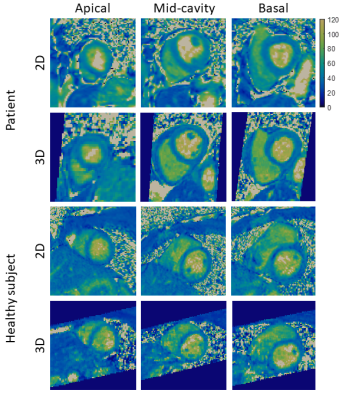
Six healthy subjects (3 males, 29±2 years-old) and three patients with suspected cardiovascular disease (2 males, 60±8 years-old) were scanned on a 3T PET-MR scanner using the proposed non-rigid motion-compensated 3D T2 mapping sequence. Data was acquired during mid-diastole using a subject-specific trigger delay, acquisition window and saturation time (longest possible). For the healthy subjects, imaging parameters included: coronal orientation, FOV=312x312x60-72mm3, 1.5mm3 isotropic resolution, bandwidth=670Hz/px, TR/TE=3.45/1.57ms, flip angle=15°, acquisition time = 9.3±1.1min. Matching imaging parameters were used for patients scans, with the exception of FOV=332x332x 64-72mm3 and resolution (1.6mm3 isotropic) with acquisition time = 8.3±0.5min. In both cases, image navigators were acquired using 14 low-flip angle (3°) lines before the 3D acquisition using the same FOV. Conventional breath-held 2D T2 maps were acquired at apex, mid and base for comparison purposes (imaging parameters for healthy subjects: resolution = 1.5x1.5x8mm3, T2-preparation pulses = 0, 28, 55 ms, flip angle=12°, bandwidth=1155 Hz/px), imaging parameters were the same for patients with exception of resolution = 1.9x1.9x8mm3.
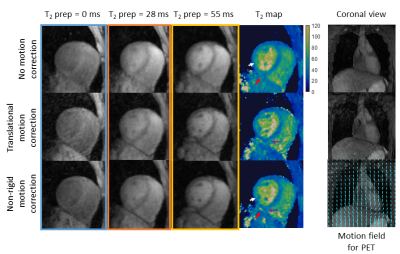
3D T2 maps were reconstructed without motion correction, translational motion correction only (TRMC) (FH and RL motion estimated from the iNAVs) and non-rigid motion correction (NRMC).
Results
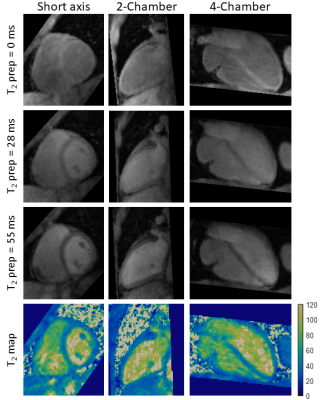
Phantom results show high correlation of the proposed approach with gold standard 2D T2-mapping (R²=0.99, Figure 2). Improvements in T2-prepared images and final T2 map image quality is seen with TRMC and further improvements with proposed NRMC as shown on Figure 3. Non-rigid motion fields are obtained with the proposed approach (Figure 3), which can be used to correct simultaneously acquired PET data. The proposed approach showed good agreement with conventional 2D T2 maps (Figure 4) while providing whole-heart coverage (Figure 5) with high isotropic resolution. In-vivo 3D T2 septal myocardium values were in agreement with conventional 2D T2 mapping (Figure 4) both in patients (3D-T2map = 40.4±2.2ms, 2D-T2map = 40.3±2.2ms) and healthy subjects (3D-T2map = 39.0±1.4ms, 2D-T2map = 38.8±1.2ms).Conclusion
An efficient free-breathing 3D whole-heart T2-mapping sequence, which provides characterisation of myocardial inflammation and non-rigid respiratory motion fields to correct simultaneously acquired PET data in a 3T hybrid PET-MR system has been proposed. Preliminary MRI-only results showed that the proposed approach achieves good image quality with T2 values comparable to reference methods in phantom, healthy subjects and patients experiments. Future studies will evaluate the proposed method in patients with cardiac sarcoidosis undergoing PET-MR.Acknowledgements
This work was supported by EPSRC (EP/L015226/1, EP/P032311/1, EP/P007619/1) and the Wellcome/EPSRC Centre for Medical Engineering (NS/A000049/1).References
1. Birnie, D. H., Nery, P. B., Ha, A. C. & Beanlands, R. S. B. Cardiac Sarcoidosis. J. Am. Coll. Cardiol. 68, 411–421 (2016).
2. Dweck, M. R. et al. Hybrid Magnetic Resonance Imaging and Positron Emission Tomography With Fluorodeoxyglucose to Diagnose Active Cardiac Sarcoidosis. JACC. Cardiovasc. Imaging 11, 94–107 (2018).
3. Bustin, A. et al. Accelerated free-breathing whole-heart 3D T2 mapping with high isotropic resolution. Magn. Reson. Med. (2019). doi:10.1002/mrm.27989
4. Prieto, C. et al. Highly efficient respiratory motion compensated free-breathing coronary MRA using golden-step Cartesian acquisition. J. Magn. Reson. Imaging 41, 738–746 (2015).
5. Henningsson, M. et al. Whole-heart coronary MR angiography with 2D self-navigated image reconstruction. Magn. Reson. Med. 67, 437–445 (2012).
6. Pruessmann, K. P., Weiger, M., Börnert, P. & Boesiger, P. Advances in sensitivity encoding with arbitrary k -space trajectories. Magn. Reson. Med. 46, 638–651 (2001).
7. Batchelor, P. G. et al. Matrix description of general motion correction applied to multishot images. Magn. Reson. Med. 54, 1273–1280 (2005).
8. Munoz, C. et al. Motion‐corrected simultaneous cardiac positron emission tomography and coronary MR angiography with high acquisition efficiency. Magn. Reson. Med. 79, 339 (2018).
9. Weigel, M. Extended phase graphs:
Dephasing, RF pulses, and echoes - pure and simple. J. Magn. Reson. Imaging
41, 266–295 (2015).
Figures