2066
Correlation between changes in cardiac iron and hepatic iron in pediatric patients with thalassemia major1MRI Unit, Fondazione G. Monasterio CNR-Regione Toscana, Pisa, Italy, 2Azienda Ospedaliera di Rilievo Nazionale "A. Cardarelli", Napoli, Italy, 3Università degli Studi della Campania Luigi Vanvitelli, Napoli, Italy, 4Policlinico "Paolo Giaccone", Palermo, Italy, 5Ospedale “SS. Annunziata”, Taranto, Italy, 6Fondazione di Ricerca e Cura "Giovanni Paolo II", Campobasso, Italy, 7Ospedale del Delta, Lagosanto (FE), Italy, 8Ospedale "V. Cervello", Palermo, Italy, 9Ospedale "A. Perrino", Brindisi, Italy
Synopsis
In pediatric thalassemia major patients who performed a baseline and a follow-up MRI study at 18±3 months the percentage changes in global heart T2* values per month were not influenced by initial hepatic iron levels and were not correlated to final hepatic iron. Moreover, the correlation between % changes in global heart T2* and MRI liver iron concentration values did not reach the statistical significance So, our data seem not supporting the hypothesis for which it is necessary to clean the liver before removing iron from the heart.
Introduction
A prospective magnetic resonance imaging (MRI) study demonstrated a good control of myocardial iron overload (MIO) in terms of prevention and treatment in children with thalassemia major (TM).1The aim of the present study was to evaluate if changes in MIO were related to baseline hepatic iron or changes in hepatic iron overload (HIO).
Methods
We considered 68 TM patients enrolled in the MIOT (Myocardial Iron Overload in Thalassemia)2 project with less than 18 years at the first MRI scan and who performed a follow-up (FU) study at 18±3 months.Myocardial and hepatic iron burdens were quantified by the T2* technique.3,4 The value of 20 ms was used as conservative normal value for the global T2* value. Liver T2* values were converted into liver iron concentration (LIC) values.5 A LIC<3 mg/g/dw indicated significant no HIO, between 3 and 7 mg/g/dw mild HIO, between 7 and 15 mg/g/dw moderate HIO, and ≥15 mg/g/dw severe HIO.
Results
Thirty-six patients were females and mean age at the time of the baseline MRI was 13.74±3.09 years.Baseline global heart T2* values were 29.72±11.21 ms and 16 (23.5%) patients showed significant baseline MIO. The percentage changes in global heart T2* values per month in the whole patient population were 0.66±1.70 and they resulted significantly higher in the 16 patients with significant baseline MIO versus the patients with no baseline MIO (1.99±2.53% vs 0.25±1.09% ms; P=0.002).
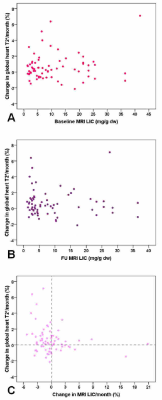
Percentage changes in global heart T2* values per month were not influenced by initial MRI LIC values (R=0.048; P=0.695) (Figure 1A) and were comparable among the 4 groups of patients identified on the basis of baseline MRI LIC values (14 no HIO: 0.29±1.12% vs 21 mild HIO: 0.75±1.56% vs 15 moderate HIO: 0.82±2.03% vs 18 severe HIO: 0.71±2.00%; P=0.876).
Percentage changes in global heart T2* values per month were not associated to final MRI LIC values (R=-0.134; P=0.277) (Figure 1B).
The correlation between % changes in global heart T2* and MRI LIC values did not reach the statistical significance (R=-0.244; P=0.067) (Figure 1C).
In patients with baseline MIO no correlation was found between % changes in global heart T2* values per month and initial MRI LIC values (R=-0.325; P=0.219) or % changes in MRI LIC values per month (R=-0.353; P=0.180).
Conclusions
In pediatric TM patients changes in cardiac iron are not correlated to baseline MRI LIC values and changes in hepatic iron. So, our data seem not supporting the hypothesis for which it is necessary to clean the liver before removing iron from the heart.Acknowledgements
No acknowledgement found.References
1. Casale M, Meloni A, Filosa A, et al. Multiparametric Cardiac Magnetic Resonance Survey in Children With Thalassemia Major: A Multicenter Study. Circ Cardiovasc Imaging 2015;8(8):e003230.
2. Meloni A, Ramazzotti A, Positano V, et al. Evaluation of a web-based network for reproducible T2* MRI assessment of iron overload in thalassemia. Int J Med Inform 2009;78(8):503-512.
3. Meloni A, Positano V, Pepe A, et al. Preferential patterns of myocardial iron overload by multislice multiecho T*2 CMR in thalassemia major patients. Magn Reson Med 2010;64(1):211-219.
4. Pepe A, Positano V, Santarelli F, et al. Multislice multiecho T2* cardiovascular magnetic resonance for detection of the heterogeneous distribution of myocardial iron overload. J Magn Reson Imaging 2006;23(5):662-668.
5. Meloni A, Rienhoff HY, Jr., Jones A, Pepe A, Lombardi M, Wood JC. The use of appropriate calibration curves corrects for systematic differences in liver R2* values measured using different software packages. Br J Haematol 2013;161(6):888-891.